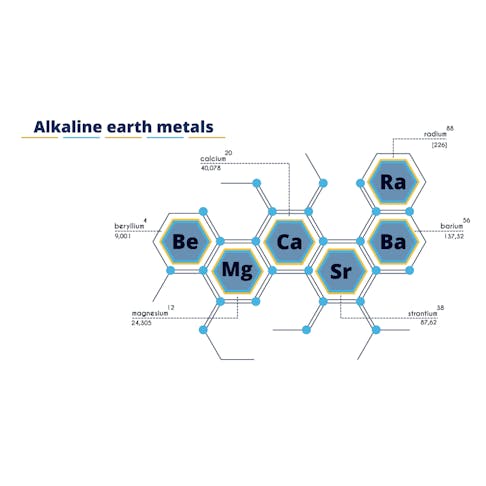
Alkaline Earth Metals: Definition, Properties, Use, and Types

Alkaline earth metals are the elements located in Group 2 of the periodic table. There are only six elements that fall within this group. These metals are characterized by their shiny, silvery-white appearance and higher densities compared to alkali metals. They have two electrons in their outer shell, which they readily lose to form +2 ions, making them highly reactive, especially with water, although less so than their Group 1 counterparts. Alkaline earth metals have a wide range of applications, from magnesium's use in lightweight alloys to calcium's role in cement and bone structure.
This article focuses on alkaline earth metals, defining them and discussing their properties, uses, and each type individually.
What Is an Alkaline Earth Metal?
An alkaline earth metal is any of six elements located in Group 2 of the periodic table. These metals are: barium (Ba), beryllium (Be), calcium (Ca), magnesium (Mg), radium (Ra), and strontium (Sr). They are called "alkaline" because they form alkaline (basic, as opposed to acidic) solutions when they react with water, and "earth" refers to their oxides which were known as earths in the past. Similar to Group 1's alkali metals, the atoms of the alkaline earth metals readily shed electrons to form positive ions (cations). Consequently, their common compounds are ionic: they are salts characterized by the metal appearing as the cation M2+, with M symbolizing any atom from the Group 2 elements. These salts are colorless unless they contain a colored anion (negative ion). Due to their reactivity, alkaline earth metals are not found free but are instead present in various minerals and ores. They are the second most reactive group of metals in the periodic table, and like the metals in Group 1, they also have increasing reactivity in the higher periods.
What Are the Different Properties of Alkaline Earth Metals?
Group 2 elements of the periodic table exhibit a unique set of properties that distinguish them from other elements. These are:
1. High Melting and Boiling Points
Compared to other groups, alkaline earth metals have relatively high melting and boiling points, which is indicative of the strong metallic bonding present within these elements. This property makes them suitable for high-temperature applications.
2. Low Density
Relative to many other metals, alkaline earth metals exhibit lower densities. This property makes them lighter, which is particularly notable in magnesium and beryllium. Their low density is a consequence of their relatively large atomic sizes combined with a moderate atomic mass.
3. Silvery-White Appearance
These metals typically have a silvery-white luster, a characteristic appearance of many elemental metals. This shiny surface is due to the metals' ability to reflect light, a property of the free electrons in their metallic bonds.
4. Good Electrical Conductivity
Like most metals, alkaline earth metals are good conductors of electricity. This property stems from their free electrons that move relatively easily through the metal under an applied electric field, facilitating electron flow.
5. Ability to Form Divalent Positive Ions
Alkaline earth metals readily lose their two outermost electrons to form divalent (+2) cations. This tendency is due to their electron configuration, aiming to achieve a stable noble gas electron arrangement. These +2 ions are characteristic features of the group's chemistry. It affects their chemical behavior and the types of compounds they form.
6. Reactivity With Water
These metals react with water to varying degrees, releasing hydrogen gas and forming hydroxides. Their reactivity with water generally increases down the group, from magnesium's slow reaction to barium's vigorous one. This behavior showcases their tendency to form compounds by losing their valence electrons. Beryllium is the only one that does not react with steam or water, even when heated to red heat (around 700–800 °C). Beryllium also forms an outer oxide layer (BeO) that protects the metal. It also lowers the reactivity of the metal.
7. Formation of Alkaline Solutions When Reacting With Water
The hydroxides formed during the reaction with water are alkaline (basic) in nature, leading to solutions that have a pH greater than 7. This property makes them very valuable in chemical processes in which pH regulation is required.
8. Ability To Form Insoluble Compounds With Sulfate Ions
Alkaline earth metals form sulfate salts, which are characteristically less soluble in water than their salts with other anions. This tendency is particularly useful in analytical chemistry for the precipitation and identification of these metals and in the treatment of wastewater to remove sulfate contaminants.
Are Alkaline Earth Metals Commonly Found as Major Components of Minerals in the Earth's Crust?
Yes, alkaline earth metals are commonly found as major components of minerals in the Earth's crust, particularly magnesium and calcium, which are abundant and play significant roles in the composition of various rocks and minerals.
Are Alkaline Earth Metals Typically Highly Reactive With Water?
Yes, most alkaline earth metals are typically highly reactive with water, though their reactivity is generally less than that of alkali metals. Magnesium has a slower reaction with water, usually requiring higher temperatures to react, while calcium, strontium, and barium react more readily at room temperature. Beryllium is an exception, as it does not react with water. The reactivity with water increases down the group, from magnesium to barium.
What Is the Use of Alkaline Earth Metals?
Alkaline earth metals are especially useful in industrial and biological applications, particularly magnesium and calcium. Magnesium, known for its lightness and strength, is extensively used in making aircraft, automotive parts, and portable electronics, as well as in fireworks and flares for its bright, white light. Calcium plays a crucial role in construction as a primary component of cement and lime, and it's also used in steel production for deoxidizing and desulfurizing. Strontium compounds are used to produce deep red colors in fireworks and have applications in medical treatments for bone strength and diseases. Barium finds its use in medical imaging, particularly in X-ray diagnostics of the gastrointestinal tract, and in the manufacturing of glass, ceramics, and lubricants. Beryllium is valuable in aerospace and defense for its hardness and light weight, as well as in electronics for its superior electrical conductivity. Radium is the only one of these elements that does not have industrial relevance. Historically, radium was used in medical treatments for cancer in the early 20th century, though its use has declined due to safety concerns.
What Are the Different Types of Alkaline Earth Metals?
There are six earth metals in total. Each of them is discussed in the sections that follow:
1. Beryllium
Beryllium (Be) is the first member of the alkaline earth metals and it is also the lightest. It has an atomic number of 4 and an electron configuration of [He] 2s². This element is not very abundant and primarily occurs in beryl, a mineral, together with aluminum and silicon. It has a shiny-white luster and a high boiling point of 2,471 °C. Beryllium is quite brittle at room temperature. Beryllium's stiffness, lightness, and superior modulus of elasticity, about 50% greater than steel at only a quarter of the weight, are some of its key properties. Although it has only half the tensile strength of alloy steels, it deflects less under load.
It can be found in over 30 minerals, including: chrysoberyl (BeAl2O4, a beryllium aluminum oxide), phenakite (Be2SiO4, a beryllium silicate), bertrandite (Be4Si2O7(OH)2, also a beryllium silicate), and beryl (Al2Be3Si6O18, a beryllium aluminum silicate). Beryllium is utilized in metallurgy, space, and nuclear applications due to its unique properties. It forms an oxide layer that protects it from further oxidation, making it valuable in structural and thermal applications, including: space mirrors, camera shutters, and semiconductor manufacturing. Additionally, it finds use in alloys, particularly with copper for non-sparking tools, in which it is used to enhance both strength and resistance. Its ability to slow neutrons benefits nuclear reactors, while its role in neutron sources has been valuable in nuclear fission research. Despite its versatility, beryllium compounds, which are generally colorless and toxic, pose health risks, such as dermatitis and chronic beryllium disease.
Beryllium’s unique properties can present some machining challenges. With it costing between $600 to $800 per pound before machining, it is important to understand its machining idiosyncrasies to avoid wasteful expenses on progressively expensive parts. Machining beryllium requires careful handling due to its: lack of ductility, similarity to powdered metals, and tendency to fracture easily. High-quality carbide tools are essential, and machining shops must adhere to strict process controls and safety protocols to manage the abrasive nature of such tools and mitigate health risks. Advancements in near-net-shape stock production aim to reduce both shipping costs and machining requirements.
2. Magnesium
Magnesium (Mg) is the eighth most abundant element in the earth’s crust. It has an atomic weight of 12 and an electron configuration of [Ne] 3s2. The element is light, also silvery-white in color, and tough. It tends to form a thin layer around itself which acts as a barrier against corrosion when exposed to air. It's known for its low density and high strength-to-weight ratio. It is the lightest structural metal, with a density of 1.74 g/cc. Like many metals that share its structure, magnesium becomes less ductile when processed at lower temperatures. Additionally, in its pure form, magnesium does not possess enough strength for most structural uses. However, adding alloying elements enhances its mechanical characteristics, rendering wrought and cast magnesium alloys suitable for applications in which a balance of lightness and strength is desired. Magnesium's remarkable lightness, decent strength, and excellent vibration-damping capabilities make it valuable in aerospace, automotive industries, and (more recently) in mobile electronics for its weight-saving benefits. Magnesium and calcium are also both used in the desulfurization of steel. Despite its wide availability and lower cost compared to beryllium, magnesium requires careful handling during machining due to its flammability. Unlike beryllium, magnesium also plays a critical role in over 300 enzymatic reactions within the human body. Magnesium costs about $8 to $12 per kg.
3. Calcium
Calcium (Ca) is the fifth most abundant element in the Earth's crust and the third most abundant metal after iron and aluminum. It has an atomic number of 20 and an electron configuration of [Ar] 4s². Calcium is a silvery-white, soft metal that tarnishes quickly in air and reacts with water. It is found in various minerals including: limestone (calcium carbonate), gypsum (calcium sulfate), and fluorite (calcium fluoride). Calcium's primary applications are in the metallurgical industry to remove impurities from alloys and in the production of cement and lime. Additionally, it plays a key role in biological processes in living organisms, such as in bone formation and muscle function. Calcium compounds are widely used in many industries; for example, calcium hydroxide is used in water treatment, and calcium phosphate is used in fertilizers. The metal itself has applications in metallurgy as a reducing agent in the extraction of other metals, such as uranium and thorium, and as an alloying agent for: aluminum, copper, lead, beryllium, and magnesium alloys. Calcium compounds are also widely used in the food industry, as dietary supplements, and in medicines. However, excessive calcium intake, whether through diet or supplements, can lead to hypercalcemia, resulting in kidney stones, renal failure, and impaired absorption of other minerals. The price for 98.5%-pure calcium metal is listed at approximately $3,662 per metric ton.
4. Strontium
Strontium (Sr), with an atomic number of 38 and an electron configuration of [Kr] 5s², is a soft, silvery-yellow metal that turns yellow when exposed to air due to oxidation. It is less abundant than calcium and does not occur freely in nature, but is found mainly in the minerals celestine (SrSO4) and strontianite (SrCO3). It also shares many properties with calcium but is heavier and more reactive. Strontium's physical characteristics, including its softness and the ability to form different crystal structures under varying temperatures, also make it unique among the alkaline earth metals. Strontium has many radioactive isotopes. The natural stable isotopes are: 84Sr, 86Sr, 87Sr, and 88Sr. There are also 31 unstable isotopes. The most environmentally significant isotopes include: 85Sr, 89Sr, and 90Sr. These isotopes pose environmental concerns due to their radioactive nature and potential health impacts.
This metal acts as an active reducing agent, quickly engaging in reactions with halogens, oxygen, and sulfur to form halides, oxides, and sulfides, respectively. In its pure form, strontium is used in the production of ferrite magnets and refining zinc. Strontium compounds, especially strontium carbonate, are used in the manufacture of fireworks and in the glass industry for color television tubes to block X-ray emission. Strontium-90, a radioactive isotope, is a byproduct of nuclear reactors and has been used in medical applications for the treatment of bone cancer due to its bone-seeking properties. Despite its usefulness, strontium has few major applications and is handled cautiously due to its reactivity. The cost of strontium metal, specifically with a purity of 99% or more, varies and often requires direct inquiry with suppliers for the most current pricing.
5. Barium
Barium (Ba), atomic number 56 and electron configuration [Xe] 6s², is a heavy, soft, silvery metal that is never found in nature in its pure form due to its reactivity. It occurs primarily in the mineral barite (barium sulfate) and witherite (barium carbonate). Barium is used in drilling fluids for oil and gas wells, and in the production of paints, bricks, ceramics, glass, and rubber. It's also used in fireworks to create green colors. Barium compounds, particularly barium sulfate, are used in medical imaging to improve the contrast of X-ray and CT scans. However, barium must be handled with care due to its toxicity.
6. Radium
Radium (Ra), with an atomic number of 88 and an electron configuration of [Rn] 7s², is a highly radioactive metal discovered by Marie Curie. It is extremely rare, occurring in uranium ores in small amounts. This metal is silvery white and quite soft. Radium readily oxidizes when exposed to air, turning from almost pure white to black. Radium is luminescent and corrodes in water to form radium hydroxide. And, even though it is the heaviest element in the alkaline-earth group it is also the most volatile.
In terms of applications, radium was once used in luminous paints for watches, clocks, and instrument dials, although this use has declined due to health concerns. Its most significant application today is in the medical field for cancer treatment in the form of radium-223, which targets bone cancer. Radium is handled with extreme care due to its radioactivity and associated health risks. The cost for radium is about $25,000 per gram.
How To Choose Which Type of Alkaline Earth Metals To Use
Choosing the right type of alkaline earth metal for your application involves considering several factors, each tied to the specific properties and uses of these elements. Here's a guide to help you decide:
- Determine what you need the metal for. Different alkaline earth metals have unique properties making them suitable for various uses, from electronics to construction materials.
- Consider reactivity. Alkaline earth metals increase in reactivity down the group. For example, magnesium is less reactive than barium.
- Evaluate hardness and density. Beryllium is harder and lighter, suitable for aerospace materials, whereas radium and barium are denser.
- Assess chemical resistance. Some alkaline earth metals, like magnesium, have good corrosion resistance when treated.
- Review temperature sensitivity. If your application involves high temperatures, consider using calcium or strontium, which have higher melting points compared to magnesium and beryllium.
- Be mindful of toxicity and radioactivity. Beryllium is toxic, and radium is radioactive, which may limit their use in consumer products.
- Some alkaline earth metals, like beryllium, are more expensive due to their rarity and extraction costs. Magnesium and calcium tend to be more affordable and widely available.
- Always review the MSDS for the metals you're considering, to understand their hazards, handling, and storage requirements.
What Type of Alkaline Earth Metals Can Be Used in Embossing?
Alkaline earth metals are rarely used in their pure form. Their compounds are usually used in machining operations like embossing, rather than the metal alone. For example, copper beryllium is easily machinable and can undergo all machining operations. The same can be true for magnesium-containing alloys.
What Type of Alkaline Earth Metals Can Be Used in Laser Engraving?
Alkaline earth metals are usually not used in laser engraving. This is because of their reactivity and properties, like magnesium’s flammability and beryllium’s toxicity. Alloys that contain alkaline earth metals can be laser engraved. Steel is an example, which can be laser engraved and laser cut.
To learn more, see our full guide on Things to Laser Engrave.
Can Alkaline Earth Metals Be Used in Laser Cutting?
No, alkaline earth metals cannot be used in laser cutting. These metals, given their reactivity and specific properties (e.g., beryllium's toxicity, and magnesium's flammability), may not be the first choice for this application without specific safety measures and technological considerations. Alloys that contain alkaline earth metals, such as magnesium and calcium, can however be used in laser cutting. An example of this is steel.
To learn more, see our How Does Laser Cutting Work guide.
What Is the Advantage of Using Alkaline Earth Metals?
Alkaline earth metals offer a range of advantages due to their distinct properties. Here are some key benefits of using these metals:
- Magnesium, in particular, is known for its low density, making it an excellent choice for lightweight constructions in automotive and aerospace applications.
- Alkaline earth metals like magnesium and beryllium provide an outstanding strength-to-weight ratio, which is essential for applications that require materials to withstand mechanical stress while remaining light.
- These metals, especially magnesium, have good thermal conductivity.
- While not as conductive as metals like copper, certain alkaline earth metals can conduct electricity well, which is useful in specific electrical applications.
- Their chemical reactivity can be harnessed in various chemical processes and applications, such as in fireworks and flares in which strontium and barium contribute vivid colors.
- The luminescent properties of radium and strontium are utilized in luminous paints, although safety concerns limit radium's use today.
- Calcium plays a critical role in biological applications due to its presence in the human body, including bone formation. It's used in dietary supplements, medical treatments, and biodegradable plastics. Magnesium is also used in biological systems.
- Elements like calcium and magnesium are abundantly available in the Earth's crust.
What Is the Disadvantage of Using Alkaline Earth Metals?
While alkaline earth metals offer several advantages, they also come with certain disadvantages such as:
- Are highly reactive, especially with water, which can limit their direct use in environments in which moisture is present. Magnesium and calcium, for example, can react vigorously with water to produce hydrogen gas, a potential safety hazard.
- Due to their reactivity, these metals can corrode quickly when exposed to air, moisture, and other corrosive agents. This necessitates protective coatings or alloys to mitigate corrosion, increasing manufacturing complexity and costs.
- Beryllium, one of the alkaline earth metals, is toxic. Its dust or fumes can cause a chronic life-threatening allergic disease called berylliosis when inhaled. Handling and processing beryllium requires stringent safety protocols.
- Some alkaline earth metals, like radium and beryllium, are rare and expensive to extract and refine. Their cost and limited availability can be prohibitive for widespread commercial applications.
- Magnesium is highly flammable, especially in powder or thin strip form, posing fire and explosion risks during handling, storage, and processing.
- Radium is radioactive and poses significant health risks, limiting its use to very specialized areas, such as medical treatments, under strict regulations.
- Several alkaline earth metals, despite being less dense and having good mechanical properties, can exhibit brittleness, especially beryllium, and magnesium, which may limit their use in certain engineering applications.
Are Alkaline Earth Metals More or Less Reactive Than Alkali Metals?
Alkaline earth metals are less reactive than alkali metals. Both groups are highly reactive compared to most other elements, but the reactivity increases down each group of the periodic table. Alkali metals, found in Group 1 of the periodic table, have a single electron in their outermost shell, which they readily lose to form a +1 charge, making them very reactive. In contrast, alkaline earth metals, located in Group 2, have two electrons in their outer shell and tend to lose both to form a +2 charge. While this still makes them quite reactive, especially with water and oxygen, the presence of two valence electrons makes them slightly less reactive than their Group 1 counterparts. This is because atoms find it harder to lose two electrons than to lose one when bonding with other atoms.
What Distinguishes Alkaline Earth Metals From Other Types of Metals?
Alkaline earth metals are characterized by their two electrons in the outermost shell, leading to their +2 oxidation state in compounds. These metals are less reactive than their Group 1 alkali metal counterparts but are still quite reactive, especially with water, forming strongly alkaline hydroxides. They are denser and harder than alkali metals, yet softer and less dense than transition metals, with higher melting and boiling points compared to alkali metals.
What Is the Difference Between Heavy Metals and Alkaline Earth Metals?
Heavy metals and alkaline earth metals differ primarily in their chemical and physical properties due to their positions on the periodic table. Heavy metals, a loosely defined group, typically include metals with a high density, atomic weight, or atomic number, and they are often associated with toxicity or environmental hazards. Examples include: lead, mercury, and cadmium. Alkaline earth metals, on the other hand, are found in Group 2 of the periodic table and are characterized by their two electrons in the outer shell, leading to a +2 oxidation state in compounds. This group is made up of: beryllium, magnesium, calcium, strontium, barium, and radium. These metals are known for their reactivity (especially with water), forming strong bases, and their use in a wide range of applications from construction to electronics. Unlike many heavy metals, some alkaline earth metals are essential to various biological processes in organisms and humans.
Summary
This article presented alkaline earth metals, explained them, and discussed their various properties and uses. To learn more about alkaline earth metals, contact a Xometry representative.
Xometry provides a wide range of manufacturing capabilities and other value-added services for all of your prototyping and production needs. Visit our website to learn more or to request a free, no-obligation quote.
Disclaimer
The content appearing on this webpage is for informational purposes only. Xometry makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through Xometry’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please refer to our terms and conditions for more information.
