The main mechanism of metallurgical degradation is corrosion. Corrosion occurs when a metal reacts with a chemical in its environment through an electrochemical process. A number of factors impact the corrosion process, including the nature of the surrounding environment (contact with air, minerals, salt water, corrosive chemicals), proximity to dissimilar metals, and the microstructure of the metal, which can lead to localized severe corrosion and cracking that can be hard to detect. Corrosion can affect any metal. However, some are more susceptible to degradation than others, depending on their reactivity and the environment to which they are exposed. This article will describe the process of metal corrosion, the factors that influence it, and its effects on metals.
What Is Metallurgical Degradation?
Metallurgical degradation is the deterioration of a material's properties over time. It can occur through four mechanisms, which are:
- Changes to a material’s microstructure
- Accumulation of damage over a period of time
- Environmental attacks, including attacks at elevated temperatures
- A mix of two or more of the above mechanisms
One very common form of metallurgical degradation is corrosion. Corrosion can affect both the physical and mechanical properties of a metal, resulting in changes in strength, ductility, and conductivity (both electrical and thermal), and can change the weight of a component.
What Occurs During Metallurgical Degradation?
During corrosion, the metal will react with its environment through an electrochemical process. The metal will give up electrons to its environment which positively charges the metal during this process. This occurs because these metals are not found in their metallic state naturally, but rather they occur naturally as a compound. After metals are processed, they slowly return to their original compound state as this form takes the least amount of energy to maintain. Corrosion will occur in many different situations using different mechanisms. Figure 1 below is a diagram of metallurgical degradation.
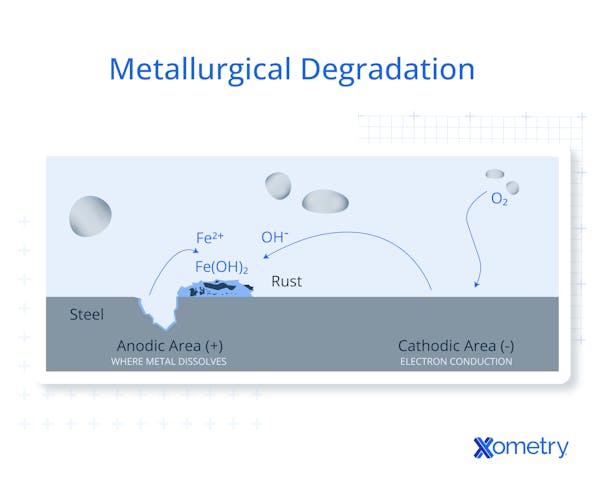
What Materials Are Affected by Metallurgical Degradation?
While materials like plastics and ceramics can also corrode, metals are the materials most generally susceptible to corrosion. However, some metals are much more susceptible than others. Platinum and gold, for example, are very unreactive and so are less prone to degradation than bare aluminum and chromium.
What Are the Factor of Metallurgical Degradation?
Many conditions factor into the acceleration of metal corrosion. These factors include:
- Exposure to gases including CO2, SO2, and SO3
- Being in contact with moisture, especially salt water
- Being exposed to salt
- Exposure to high-temperature environments
- Lack of protection from oxide layers, for example, when aluminum corrodes, it forms a protective oxide layer. Conversely when steel corrodes the outer layer falls off exposing new metal in the process.
How Does Metallurgical Degradation Works on Static Equipments?
“Static equipment”, also known as “fixed equipment”, is a term used in the oil and gas industry when referring to pressure vessels, piping, and heat exchangers. The term does not refer to pumps and valves which contain moving parts. Static equipment may corrode through the “erosion corrosion” process where fluids can wash away corrosion products, leaving the unprotected metal exposed. This is accelerated if the fluid is fast-moving, turbulent, and/or containing particles causing increased wear. Static equipment must be periodically inspected for any signs of corrosion.
How Does Metallurgical Degradation Works on Gas Turbines?
Hot corrosion is a special form of corrosion occurring in gas turbines where contaminants in the hot gas of the gas turbine react with metals resulting in corrosion. The high temperature of the gas can accelerate the corrosion process. The chemical attack during hot corrosion can cause the build-up of scale on metal components within the engine.
How Does Metallurgical Degradation Works on Electrode Surfaces?
Degradation of electrode surfaces within a battery occurs as a cell provides power to a load. Free electrons must flow around a circuit to create a current. Two electrodes are placed in a solution to induce an electron flow. Then an oxidation reaction will occur with the iron at the expense of a reduction reaction with the copper when the iron and copper electrodes are connected. Rephrased, the iron will dissolve releasing an electron so the copper solution can gain an electron and attach to the copper. For more information, see our guide on Thermal Conductivity.
How Is Metallurgical Degradation Varies in Different Types of Metals?
Corrosion affects metals very differently depending on their properties. The electromotive series defines the reactivity of all metals which directly relates to the metals’ susceptibility to corrosion. For example, zinc and magnesium are highly reactive and are therefore more susceptible to corrosion than platinum and gold, which are more resistant to corrosion. The adherence of an oxide layer also affects a metal's protection from corrosion. Aluminum is less susceptible to severe corrosion as it forms an oxide layer that prevents further corrosion from taking place. For steel, however, the oxide layer will fall off leaving more exposed metal. Alloys such as steel are protected from corrosion with the addition of chromium which forms a chromium oxide layer.
How Long Does It Take for a Steel Can To Degrade?
A plain carbon steel surface can start to rust within a matter of hours from first exposure to oxygen and moisture, for example, after a protective coating is scratched. However, steel can take anywhere from 50 to 100 years to fully degrade, depending on the thickness of the material and the environment in which it is left. The moisture content, pH, and chloride level of the soil present three variables that affect the rate of corrosion of steel. In order to corrode, steel requires moisture and oxygen. Aluminum, on the other hand, takes a lot longer to decompose — on average around 200 years. For more information, see our guide on Steel Metals.
What Is the Role of Corrosion in Degrading Metal Components?
Corrosion is a primary mechanism for the degradation of both aesthetic and physical properties. Corrosion achieves this mainly through the thinning of metal which occurs due to the corrosion product falling off of the metal. The thinning of metals will lead to a reduction in load-bearing capacity, resulting in failure. Corrosion will usually reduce the material and therefore, mechanical properties in the local area. This can happen through any of the corrosion mechanisms including erosion-corrosion, crevice corrosion, pitting corrosion, etc. However, it will usually lead to stress corrosion cracking because the area of material loss is a stress center.
Can Salt Water Degrade Metals Than Gas?
Yes, saltwater degrades metals faster than gas does. The level of chloride ions present in saltwater is a very aggressive cause of corrosion. The extent and speed of corrosion are much faster when metal is exposed to saltwater than to gasses such as oxygen in the atmosphere.
Can Oil Preserve Metal From Degrading?
Yes, covering a metal in oil protects it from the mild corrosive environments (for example oxygen or water) that would otherwise, degrade it. This protective barrier reduces exposure of the metal surface to any of these external corrosive agents from attacking the material.
Is Corrosion a Physical Change?
No, corrosion is an example of a chemical change. When a material is exposed to air, the metal surface reacts with oxygen in the atmosphere, and changes occur on the surface of that material as a result. This is a chemical change, as there is a new product that has been formed. For example, iron becomes iron oxide or rust.
Does Hard Metals Means Degrades Longer?
No, the strength or hardness of a metal is not correlated with how fast it may corrode. For example, pure iron is a hard metal that corrodes extremely quickly. On the other hand, gold corrodes very slowly and is a soft metal. For more information, see our guide on Durometer Hardness.
Summary
This article presented metallurgical degradation, explained it, and discussed how it affects metals and how it occurs. To learn more about metallurgical degradation, contact a Xometry representative.
Xometry provides a wide range of manufacturing capabilities, and other value-added services for all of your prototyping and production needs. Visit our website to learn more or to request a free, no-obligation quote.
Disclaimer
The content appearing on this webpage is for informational purposes only. Xometry makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through Xometry’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please refer to our terms and conditions for more information.

