Polymers and metals are two of the most widely used material types in manufacturing. This guide compares their structures, properties, applications, and costs to help engineers choose the right one.
Engineers and materials scientists often face the choice between using polymers or metals in their work. Both classes of materials have unique properties and applications. In contrast to polymers, which are large molecules made up of repeating units, metals are naturally crystalline and composed of metallic elements. Polymers are useful in many applications, such as packaging materials and medical implants. Metals, on the other hand, are necessary for industries like construction and electronics due to their conductivity and strength. Due to fundamental structural differences, these materials have different mechanical, electrical, and thermal properties. This article will discuss each material's advantages, traits, and applications as well as how they differ.
What Is a Polymer?
A polymer is a large molecule made of repeating subunits called monomers. These monomers are linked by chemical bonds to form long chains that can be linear, branched, or cross-linked. Natural examples include silk, proteins, and DNA, while synthetic polymers include plastics and rubbers. Their structural diversity enables a broad range of applications in packaging, electronics, and medicine.
What Is Polymer Made Of?
Polymers are made up of monomers — small molecules that chemically bond to form long molecular chains or networks. Most synthetic polymers are hydrocarbon-based, with carbon and hydrogen atoms forming the molecular backbone. The type and arrangement of monomers determine the polymer’s physical behavior and properties.
What Is the Importance of Polymers?
Polymers play a significant role in modern industries, including packaging, textiles, electronics, and medicine. Their advantages include affordability, lightweight design, durability, and flexibility. Some polymers are biodegradable and thus contribute to sustainability efforts by reducing waste and energy use during manufacturing processes.
What Are the Properties of Polymers?
Among the distinctive characteristics of polymer materials are:
- High Strength and Toughness: Materials like polyethylene (PE) exhibit strong resistance to deformation due to long-chain molecular entanglement.
- Low Density: Their lightweight nature makes polymers ideal for applications requiring mass reduction.
- Electrical and Thermal Insulation: Polymers have low conductivity, making them effective insulators.
- Chemical Resistance: Many polymers can withstand aggressive chemicals that degrade other materials.
- Biocompatibility: Certain polymers are compatible with biological systems and are used in implants and drug delivery devices.
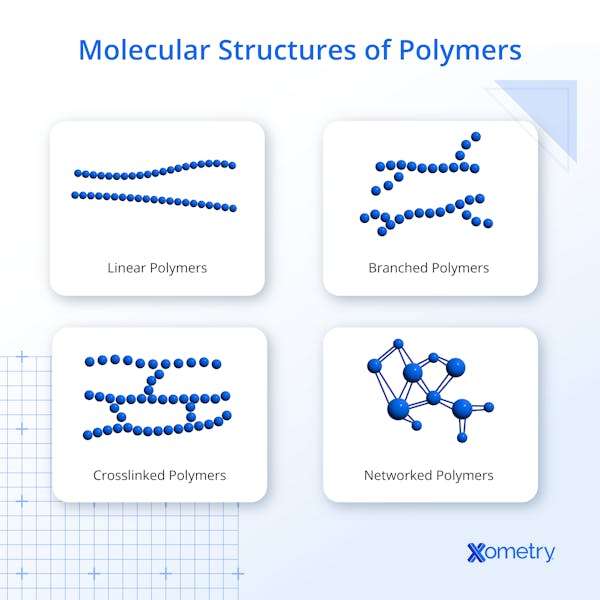
What Is the Structure of a Polymer?
All polymers share a structure composed of repeating monomer units connected by covalent bonds. The configuration — linear, branched, or cross-linked — depends on the monomers used and polymerization conditions. This structure influences key properties like flexibility, melting point, and toughness.
What Is the Advantage of Polymer Compared to Metal?
Compared to metals, polymers offer:
- Low Weight: Their reduced density makes them suitable for lightweight designs.
- Corrosion Resistance: Polymers do not rust or degrade in harsh environments.
- Insulating Properties: Their low conductivity supports electrical and thermal insulation.
- Flexible Design Options: Polymers can be molded into intricate or unconventional shapes.
What Are Examples of Polymers?
Polymers are found in a wide range of products and materials, including plastics, fibers, adhesives, and coatings. Here are a few examples of polymers:
- Polyethylene: This is one of the most common plastics, making up products like plastic bags, water bottles, and food containers. Repeated ethylene monomers make up versatile polyethylene chains.
- Polystyrene: Commonly used in foam cups, packaging, and toys. It is formed by repeatedly linking styrene monomers.
- Polyvinyl Chloride (PVC): This plastic is utilized in products like electrical cables, medical equipment, and building materials. Vinyl chloride monomers are the building blocks for the polymer known as PVC.
What Is Metal?
A metal is a substance composed primarily of metallic elements, such as iron, copper, aluminum, and gold. Metals are typically strong, shiny, ductile, malleable, and excellent conductors of heat and electricity. These properties make them essential to industries like construction, transportation, and electronics.
What Is Metal Made Of?
Metals consist of atoms classified as metallic elements on the periodic table. These elements are abundant in the Earth’s crust and possess unique bonding behavior that gives metals their characteristic properties.
What Is the Importance of Metals?
Metals are valuable because of their distinctive physical and chemical characteristics, which make them necessary in many applications. They are strong and resilient, excellent heat and electricity conductors, and easily moldable. Metals are employed in nearly every field of manufacturing, including construction, transportation, electronics, and medicine.
What Are the Properties of Metal?
The properties of metals include:
- High Electrical Conductivity: Metals are excellent conductors of electricity, which makes them essential in electrical wiring and circuitry.
- High Thermal Conductivity: Metals also conduct heat well, making them important in heating and cooling systems.
- High Ductility: Metals can be stretched and shaped without breaking, allowing metal items to be manufactured in various forms and shapes.
- High Malleability: Metals can be easily hammered, pressed, or rolled into thin sheets or other shapes without breaking.
- High Melting and Boiling Points: Most metals have high melting and boiling points, which makes them useful in high-temperature applications.
What Is the Structure of Metal?
Metals are structured as crystal lattices — tight, repeating atomic patterns — held together by metallic bonds. These bonds involve delocalized electrons that move freely between atoms, enabling conductivity, ductility, and strength.
What Is the Advantage of Metal Compared to Polymer?
Metals offer several performance advantages over polymers:
- Strength and Durability: Metals withstand greater loads and environmental stress.
- High Conductivity: Metals efficiently conduct heat and electricity.
- Hardness: Their rigid atomic structure resists wear and impact.
- Thermal Resistance: Metals tolerate higher temperatures than most polymers.
What Are Examples of Metals?
Here are some examples of metals:
- Iron: Iron is a typical metal that has been used in construction, tools, and weapons for millennia. It is a crucial component of steel, which is one of the modern world’s most fundamental structural materials.
- Aluminum: A strong, lightweight metal used in electronics, construction, packaging, and particularly in aircraft due to its favorable strength-to-weight ratio.
- Gold: For thousands of years, people have prized gold as a precious metal because of its beauty and scarcity. It serves as a store of value and appears in coins and jewelry. Gold is also used in electronics and medical equipment due to its excellent conductivity and biocompatibility.

Which Is Better, Polymer or Metal?
There is no definitive answer as to whether polymers or metals are better; it depends entirely on the application. Metals are durable and strong, making them ideal for load-bearing or high-stress uses. Polymers are lightweight, corrosion-resistant, and easier to shape, which makes them ideal for flexible or cost-sensitive designs. The choice should be guided by functional requirements and operating conditions.
Is a Polymer a Metal?
No, polymers and metals are fundamentally different materials. Polymers are composed of large molecules made of repeating monomer units, while metals are elemental solids with crystalline structures and metallic bonding. Polymers are often used in plastics and insulation, whereas metals are used for strength and conductivity.
Is Polymer Stronger Than Metal?
Generally, metals are stronger than polymers in terms of tensile strength and structural toughness. However, some engineered polymers, such as Kevlar® and carbon fiber-reinforced composites, can outperform certain metals in specific applications. Metals still remain the preferred choice for high-load, high-stress environments like construction and aerospace.
Is Polymer Cheaper Than Metal?
Generally, polymers are less expensive to produce and process than metals. However, high-performance polymers can be more costly than common metals like steel or aluminum. Material cost is influenced by production methods, required properties, and manufacturing volume.
Do Polymers Break Down Easily?
Some polymers, like polylactic acid (PLA), degrade quickly in composting environments, while others, like polyethylene, are highly durable and can persist for centuries. Degradability depends on both the polymer’s chemical structure and environmental exposure.
What Is the Difference Between Polymer and Metal in Terms of Tensile Strength?
Metals generally possess higher tensile strength due to their crystalline atomic arrangement and metallic bonding. This structure allows stress to be distributed effectively. However, specialized polymers like carbon-fiber-reinforced plastics can rival or exceed the strength of certain metals in specific cases.
What Is the Difference Between Polymer and Metal in Terms of Weight?
Polymers typically have lower density than metals, making them much lighter by volume. This weight difference makes polymers ideal for lightweight or portable designs, although specific comparisons should always consider the exact materials involved.
What Is the Difference Between Polymer and Metal in Terms of Hardness (Durometer)?
Metals are generally harder than polymers due to their tightly packed crystalline structure and strong metallic bonds. Polymers usually have lower durometer hardness ratings, though their hardness can be modified through fillers, reinforcements, or processing techniques. For more information, see our guide on Durometer (Hardness).
What Is the Difference Between Polymer and Metal in Terms of Chemical Structure?
Polymers are made from repeating organic monomers joined by covalent bonds. Metals consist of atoms arranged in a lattice, bonded by shared delocalized electrons. These distinct bonding types result in fundamentally different behaviors in strength, conductivity, and flexibility.
What Is the Difference Between Polymer and Metal in Terms of Application?
Polymers are synthetic materials that are lightweight, flexible, and have good insulating properties. Consumer goods, textiles, and packaging are just a few examples of their uses. On the other hand, metals are strong, long-lasting substances that frequently get used in the building, manufacturing, and transportation sectors.
What Is the Difference Between Polymer and Metal in Terms of Ductility?
Metals are more ductile than polymers due to the nature of metallic bonding, which allows atoms to slide past each other without breaking. Polymers, by contrast, are often brittle or exhibit limited plastic deformation, depending on their molecular structure and temperature. For more information, see our guide on Ductility.
What Is the Difference Between Polymer and Metal in Terms of Production?
When monomers are joined to form long chains through the process of polymerization, a material known as a polymer is created. In contrast, metals are created by mining and refining earthbound ores, which are then melted and shaped as desired using casting, forging, or extrusion techniques.
Summary
This article compared polymers and metals, explained what each is, and discussed their differences in manufacturing. To learn more about polymers vs. metals, contact an Xometry representative.
Xometry provides a wide range of manufacturing capabilities and other value-added services for all of your prototyping and production needs. Visit our website to learn more or to request a free, no-obligation quote.
Disclaimer
The content appearing on this webpage is for informational purposes only. Xometry makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through Xometry’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please refer to our terms and conditions for more information.


