Thanks to its long-lasting effect, low maintenance, and ability to add durability to a material, manufacturers love to anodize any metal that can possibly be anodized. It’s a no-brainer really; it’s cost-effective and makes the metal look nicer, too. Let’s have a look at exactly what it is and how it works.
What is Anodizing?
Anodizing is an electrochemical technique that improves both the functionality and appearance of nonferrous metals, most commonly aluminum and its alloys, but also copper, titanium, manganese, magnesium, zinc, and stainless steel (each material reacts differently to it, but all get the benefits). The magic is in the thin and porous oxide layer created on the metal’s surface during the very controlled anodizing process that protects it from corrosion, oxidization, and wear and tear.
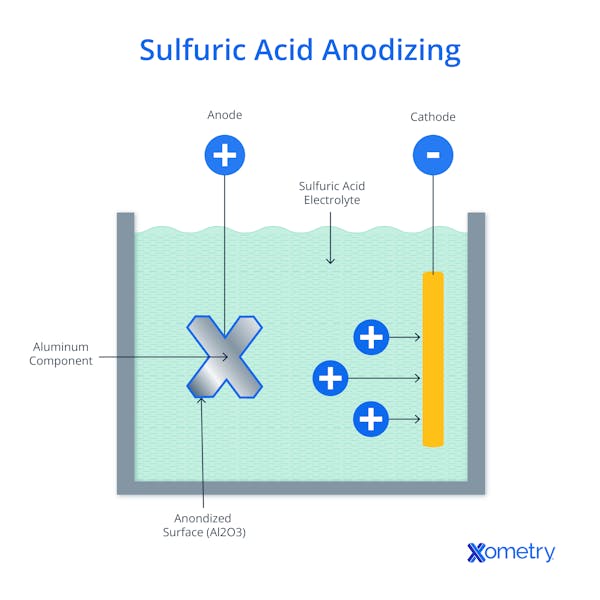
As you can see in the above image, the process involves submerging a metal in an acid bath solution and applying an electric current that drives a chemical reaction, and this is what forces the protective layer to build up. To get a little more technical, the metal to be treated acts as the anode in the electrochemical circuit. When an electric current passes through the system, negatively charged oxygen ions (anions) from the acidic electrolyte are drawn to the anode—these oxygen ions bond to form the oxide layer with the aluminum surface.
At the same time, positively charged hydrogen ions (cations) in the electrolyte are reduced (gain electrons) at the cathode, which is typically made of T-6063 aluminum alloy or other inert, conductive materials. The cathode and anode are connected to an external power source that provides the necessary driving force to sustain reactions and complete the circuit. The thickness of the layer can be customized and tailored to specific needs by changing the type of acid used, voltage, temperature, and process duration.
The process makes the material harder, stronger and a lot more durable than the metal underneath, and also smoother so it can be dyed in various hues and painted more easily—all while keeping the material’s natural appearance. But there aren’t all that many color options due to the chemicals used, and these coatings are more prone to scratches that can’t be discreetly retouched (touch-ups are annoyingly visible).
When Was Anodizing First Used?
The method dates back to 1923 when G.D. Bengough and J.M. Stuart, two British inventors, filed a patent application for the method. An early use was to make seaplane parts made from an early aluminum called Duralumin, which is resistant to saltwater corrosion. In 1927, a new sulfuric acid-based process was patented, and it’s still the most common method used to this day. From the 1930-40s onwards, anodizing started being used in military, aerospace, and marine applications, and in other industries with heavy-duty demands, and, a bit later on, in architecture and electronics, too.
How Does Anodizing Work?
Anodizing is the process of creating a carefully controlled, tightly adherent layer of oxide on an aluminum or other nonferrous metal surface. The process works by using an electric current to force an oxide layer to build up on the workpiece. The workpiece functions as an anode, attracting negatively charged oxygen ions. These oxygen anions come from the acidic electrolyte. The driving force to create the negatively charged ions comes from electrons emitted at the cathode, which is usually made of T-6063 aluminum alloy, though it can be made of other inert, conductive materials. Positively charged hydrogen ions created from the electrolyte at the same time as the negatively charged oxygen ions are reduced (take on electrons) at the cathode. The circuit is completed by a power source and wiring to the anode and cathode outside the anodizing tank.
What Is the Importance of Anodizing?
Anodizing provides many key benefits to metals. The most important benefits are increased wear resistance, increased corrosion protection, and aesthetic improvements. Anodizing creates a thin layer of oxide on the surface of a metal, which is much more resistant to wear and tear, as well as protecting against corrosion. The surface created by the anodizing process also makes metals more suitable for dyeing and painting, allowing metal surfaces to be transformed into a variety of colors. Unlike other metallic finishes, anodizing allows the metal to retain its metallic appearance.
What Types of Materials Can be Anodized?
The most common anodized materials are aluminum and aluminum alloys, but the anodizing process can be applied to other metals as well, such as copper, titanium, manganese, magnesium, zinc, and stainless steel.
Which Material Is Anodized the Most?
Aluminum is the most commonly anodized material. Aluminum anodizing is a popular preventive measure that protects the metal's surface from corrosion and wear. Anodized aluminum develops a surface that is three times tougher than that of regular aluminum, and that will not flake, chip, or peel, even after being colored. The product won't ever rust, tarnish, or weather because of the controlled aluminum oxidation layer produced by anodizing.
What Are the Anodizing Colors?
Anodized surfaces can be dyed to produce any shade. However, not all dyes are equal, and there are a few colors that are used more commonly than others. Red, blue, green, black, yellow, purple, and orange are some of the most commonly used anodizing colors. Their popularity is mostly due to their low cost and ease of manufacture.
How to Anodize Aluminum
The steps involved in anodizing aluminum are pretreatment, anodizing, coloring, and then sealing. We’ve already covered anodizing above, so here are how the other steps factor into the overall process:
1. Pretreatment
The surface of the metal has to be very clean and even before the process can start. To remove any dirt, oils, grease, and other contaminants, mechanical methods like polishing, shot peening, and sandblasting can be used, but this is done many times with chemical pre-treatments, such as:
- Degreasing, where the metal is plunged in a grime-busting solution to remove any impurities, then rinsed in hot water.
- Etching, which uses a bath of caustic soda (sodium hydroxide) to even out texture and remove minor imperfections. This breaks off into two further branches—light etching for a satin, matte finish, and bright dip anodizing that uses phosphoric/nitric acid for a more luminous look.
- Desmutting treats the material with an acid solution, often nitric acid, to remove any residual smut (a combination of impurities and alloying elements). This helps the oxide layer stick evenly across the metal and gives it a smooth surface.
2. Coloring
The porous oxide layer formed in the anodizing step (just before this one) allows for easy color dying, which appeals to custom manufacturers—it’s not a necessary step but is used a lot for branding and decorative purposes. While there are many color options, the final result will depend on factors like the type of dye used, the bath’s chemical composition, and how long it’s immersed. The coloring methods are:
- Electrolytic: Dipping the material in a tin, cobalt, nickel, or other inorganic metal salt solution and then zapping an electric current through it. These salts create the colors by oxidizing and settling into the layer’s pores.
- Dip: Immersing the material in a solution with inorganic or organic dyes. The porous layer will have absorbed the dye so rinsing the surface is a must to avoid any further reaction from happening—but this option could mean the color fades quickly, and it isn’t UV-resistant.
- Advanced technologies: These are on the up and allow for custom color matching. They’re not yet as common as the above two but include lasers, software, and nanotechnology.
3. Sealing
Sealing is really important to the overall anodizing process because it removes any remaining porosity on the surface so that the piece truly stays protected from harsh external environments and elements, scratches, and staining. There are three ways it’s done:
- Cold sealing with room-temperature solutions (typically nickel or cobalt salts)
- Hot sealing, which involves boiling water or steam to hydrate and seal the oxide layer
- Hybrid sealing, which is a combination of the hot and cold methods
What Are the Types of Anodizing?
Although there are some specialized anodizing types available used for more niche purposes (i.e., phosphoric acid or titanium), these are the three main ones that are chosen based on the application, material, and desired end result:
1. Type I - Chromic Acid Anodizing
- Electrolyte: Chromic acid
- Layer thickness: 0.00002 to 0.0001 inches (0.5 to 2.5 microns)
- Properties: Excellent bonding, non-conductive, electrically insulating, minimal dimensional impact, compatible with complex shapes
- Applications: Adhesive applications, paint and coating primer, aerospace components, welded parts
This type produces the thinnest oxide layer of the three, but if it’s properly sealed, it can give your metal as much corrosion resistance as the thicker layers formed by the other two. These coatings absorb less dye, resulting in a grayish look that limits its use for decorative applications. But you can dye the coating black, which is great for things like optical component housings where protection and light absorption are needed.
2. Type II - Sulfuric Acid Anodizing
- Electrolyte: Sulfuric acid
- Layer thickness: 0.0002 to 0.001 inches (5 to 25 microns)
- Properties: Highly porous, hard, abrasion and corrosion resistant, more affordable, compatible with a wider range of aluminum alloys
- Applications: Optical and electronic components, hydraulic valve bodies, electronic and computer enclosures
This is the most widely used type; it creates a more rigid coating than Type I and is more affordable than both the other methods because it doesn’t need as much energy and time or as many chemicals. Also, treating waste from sulfuric acid anodizing is simpler and less expensive than waste management for chromic acid types. It can absorb dyes (which are available in many different shades) before it’s sealed, thanks to its porous layer. Post-dye sealing will minimize color fading under regular use but it won’t be able to stop the color from wearing off in prolonged UV exposure. The final look is clear and natural, making it an excellent base for dyeing, but it can also be left uncolored.

3. Type III - Hard Coat Anodizing
- Electrolyte: Sulfuric acid-based
- Layer thickness: 0.0005 to 0.002 inches (12.5 to 50 microns)
- Properties: Superior wear and corrosion resistance, non-conductive, insulating
- Applications: Electrical system components, valves and pistons, sliding parts, gears, joint swivels, blast shields, electrical insulation, high-friction and high-load applications
Also called hard or hard coat anodizing, this is done under tightly controlled conditions as it creates a denser, sturdier oxide layer than standard sulfuric acid anodizing that can extend the service life of components. It can be combined with lubricants to improve the performance of sliding parts, reducing wear and friction, and the thickness and uniformity of the coatings make them great for refurbishing worn components or remanufacturing parts that fall out of specification.
Anodizing is a controlled oxidation of the outer surface of aluminum. This oxide layer is almost like a thin ceramic. It creates a protective barrier that is corrosion and scratch resistant — even more durable if you choose Type 3 anodizing that is best for high-wear products. Another benefit is the ability to pigment that oxide layer with colors. Black is the most popular but there are also other striking pigments like blue, gold, purple, etc.Greg PaulsenDirector, Applications Engineering
| Benefits | Description |
|---|---|
Benefits Corrosion protection | Description The thin layer formed by anodizing helps protect metals against corrosion. |
Benefits Durability | Description Anodizing causes the surface of metals to be more resistant to wear. |
Benefits Improved Appearance | Description Anodizing transforms the surface of a metal and leaves it more suitable for dyeing or painting. |
Benefits of Anodizing
| Limitations | Description |
|---|---|
Limitations Color selection | Description Color options are severely constrained due to the chemicals utilized in the anodizing process. |
Limitations Touch-ups are visible | Description Colored anodized coatings are susceptible to scratching. The scratches cannot be easily touched up. |
Limitations of Anodizing
Frequently Asked Questions on Anodizing
Is Anodizing a Chemical Process?
Yes, anodizing is an electrochemical process where a part (an anodically connected metal) is immersed in an acid electrolyte solution and an electric current is passed through the medium.
Why Is Aluminum the Most Commonly Anodized Metal?
Aluminum is the most widely used metal for industrial and commercial use—even more than steel and titanium—which explains why it’s the most commonly anodized. As well as all the benefits mentioned above, anodizing gives the metal a surface that’s three times tougher than if it were left untreated. It doesn’t flake, chip, or peel (unlike some other coatings), even when colored or dyed.
What Colors Can Be Achieved With Anodizing?
The most common colors you can get are red, blue, green, black, yellow, purple, and orange—not because they’re the nicest colors available, but because these are generally the most cost-effective and are easy to produce shades. You can also get gold, bronze, nickel, and transparent finishes. It’s worth repeating that the final appearance will depend on several factors, like the alloy composition, the layer’s thickness, and dye quality.
How Xometry Can Help
Processing your metal parts is more than possible at Xometry and we have a long list of manufacturing services you can get instant quotes for depending on your needs, including for anodizing and other customized services. Take a look at our plastic 3D printing, plastic extrusion, and plastic laser cutting pages for more information. You could always reach out to one of our representatives, who will be more than happy to assist, or go straight to requesting your free, no-obligation quote on any service.
Disclaimer
The content appearing on this webpage is for informational purposes only. Xometry makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through Xometry’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please refer to our terms and conditions for more information.


