Polymers are materials composed of repeating chains of smaller molecular units called monomers. Polymers can be naturally occurring (such as cellulose, latex, and rubber) or synthetic (like nylon, polyethylene, and polypropylene). The terms “polymer” and “plastic” are often used interchangeably, but there are noticeable differences between the two. Plastics are mostly synthetic polymeric materials, commonly derived from crude oil and natural gas, and are a subset of polymers. Therefore, while all plastics are polymers, not all polymers are plastics. Differences between the two also include recyclability, flexibility, and strength. This article will define polymers and plastics and discuss the differences between the two types of materials.
What Is a Polymer?
A polymer is a natural or synthetic substance composed of repeating molecular units, or monomers, that are chemically bonded into long chains. Polymers can be characterized as homopolymers (polymers comprised of one type of monomer) or as copolymers (polymers comprised of two or more monomers). Polymers form the basis of many materials, from proteins and nucleic acids in organisms to synthetic products like plastics and rubber.
What Is a Polymer Made Of?
A polymer is made of long chains of repeating monomer units that are chemically bonded together. These monomers can be simple, made of only a few atoms, or more complex functional groups. The chemical composition and size of the individual monomers determine how the polymer interacts with itself and its surrounding environment.
What Are the Properties of a Polymer?
The properties of polymers differ based on their chemical composition. However, the general properties of polymers are listed below:
- Longer chain lengths and higher crosslink density generally increase the tensile strength of polymers.
- Longer chains and stronger intermolecular forces generally lead to higher melting or softening points in polymers.
- Many polymers are chemically resistant due to their low reactivity.
- Polymer backbones are held together by covalent bonds, while secondary interactions such as hydrogen bonding, van der Waals forces, or ionic interactions can occur between chains.
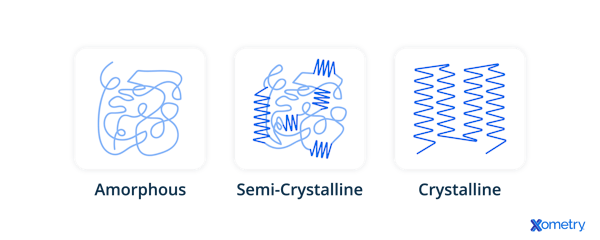
- Most polymers are either semi-crystalline (with both ordered and disordered regions) or fully amorphous.
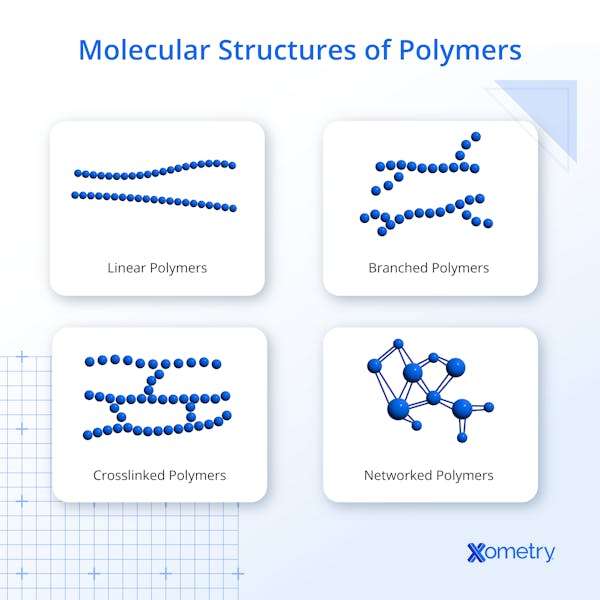
What Is the Structure of a Polymer?
Polymers can have different molecular structures: linear, branched, cross-linked, or networked. Cross-linked and networked polymers are closely related, since both involve bonds between parallel chains, but they are sometimes described separately depending on the density and connectivity of those bonds. Linear polymers consist of a chain of monomers, typically called the backbone in branched and cross-linked polymers. Bonded to the backbone are pendant groups, which can include atoms such as hydrogen, chlorine, or fluorine, or more complex functional groups. These pendant groups hang from the carbon chain like charms on a bracelet. When pendant groups connect to other parallel chains, the result is a network or cross-linked polymer. In general, higher crosslink density increases stiffness and strength, while fewer or longer crosslinks allow greater flexibility.
What Is the Advantage of Polymer Compared to Plastic?
There are many advantages of using polymer compared to plastic. The list below describes some of the advantages:
- Natural polymers are not derived from crude oil.
- Some natural polymers may release fewer harmful byproducts when burned compared to synthetic plastics, though this depends strongly on the material.
- Many natural polymers are more biodegradable and sustainable than synthetic plastics.
What Are Examples of Polymers?
Polymers are found everywhere. They are present in living organisms and in many natural materials, though not typically in minerals. Some examples of polymers are listed below:
- Cellulose and lignin are natural polymers that form the basis for plants and trees.
- Nylon, polypropylene, and polyethylene are synthetic polymers that are classified as thermoplastics.
- Elastomeric materials like rubber and latex.
What Is Plastic?
Plastics are synthetic polymeric materials, most commonly derived from petroleum. They are produced mainly through addition polymerization or condensation polymerization processes. In these processes, fractions from oil and natural gas, such as ethane and propane, are cracked to produce monomers like ethylene and propylene. The monomers and a catalyst are mixed to form a polymer. This mixture is then extruded, cooled, and cut into pellets, which are then shipped to plastics fabrication companies around the world.
What Is Plastic Made Of?
Plastics are primarily made from petroleum and natural gas, though coal derivatives and bio-based sources such as cellulose can also be used. Oil is a hydrocarbon that, when refined, is separated into fractions that differ in molecular size and structure. Naphtha, a fraction obtained from oil refining, is one of the main feedstocks for plastics production, especially for producing ethylene and propylene through cracking.
What Are the Properties of Plastic?
There are two types of plastics: thermoplastics and thermosets. The properties of these two types of plastics differ depending on the particular thermoplastic or thermoset. However, a list of general properties of plastics is shown below:
- Thermoplastics soften or melt when heated above their glass transition or melting temperature and can usually be cooled and reheated multiple times without significant chemical change.
- Thermosets are plastics that form irreversible cross-linked networks during curing and do not melt upon reheating.
- Many plastics are chemically stable.
- Most plastics are electrical insulators and have low thermal conductivity compared to metals.
- They often provide a high strength-to-weight ratio.
What Is the Structure of Plastic?
Because plastics are polymers, they can have linear (like PVC), branched (like low-density polyethylene), or cross-linked (like bakelite and melamine) structures. The chemical composition of the monomers that form the plastic determines its structure and type of polymer. Plastics can be amorphous, with disordered molecular chains, or semi-crystalline, containing both ordered crystalline regions and amorphous regions.
What Is the Advantage of Plastic Compared to Polymer?
There are many advantages of using plastic compared to polymers. The list below describes some of the advantages:
- Many plastics can be produced at relatively low cost compared to natural polymers or specialty synthetic polymers.
- Plastics can be manufactured efficiently in large volumes using processes like injection molding and extrusion.
- Plastic has a high strength-to-weight ratio.
- Plastic is versatile and used in many applications, from consumer goods to industries such as automotive, medical, aerospace, and heavy machinery.
What Are Examples of Plastics?
Some examples of plastics are listed below:
- Nylon
- Low-density polyethylene (LDPE)
- PVC
- Polyoxymethylene (POM)
- Polypropylene (PP)
In my experience, choosing between polymers and plastics is rarely about which is better overall, but about matching the material to the job. The right selection depends on strength, cost, sustainability, and how the part will actually be used.Audrius Zidonis, Principal Engineer at Zidonis EngineeringNote from the Editor
Which Is Better for 3D Printing, Polymer or Plastic?
Whether polymers or plastics are better for 3D printing largely depends on the application of the printed part. Polylactic acid (PLA) is a popular biodegradable polymer used for 3D printing of short-life-cycle, non-load-bearing parts, such as hobbyist prints. ABS, a thermoplastic polymer lauded for its strength, durability, and impact resistance, is often used to 3D print toys, electronic enclosures, and automotive parts. The ease of 3D printing depends on the specific polymer used. Some, like PLA and ABS, are highly processable, while others may require specialized printers or conditions.
What Are the Advantages of Using Polymer in 3D Printing?
The advantages of using polymers in 3D printing are listed below:
- Natural polymers like gelatin and alginate can be used in bioprinting to create hydrogels for cell culture and tissue engineering.
- Natural polymers can be blended with plastics in 3D printing filaments to reduce environmental impact and improve biocompatibility.
What Are the Advantages of Using Plastic in 3D Printing?
The advantages of using plastic in 3D printing are listed below:
- 3D printing with plastics can produce strong, lightweight parts.
- In some applications, strong engineering plastics like nylon or polycarbonate can replace metal parts, particularly for lightweight prototypes or non-structural components.
- 3D printing with plastics is an effective method for proof of concept and prototyping.
What Are the Disadvantages of Using Polymer in 3D Printing?
A limitation of polymers in 3D printing is that only a small subset of natural and synthetic polymers is printable with current technologies.
What Are the Disadvantages of Using Plastic in 3D Printing?
The disadvantages of using plastic in 3D printing are listed below:
- Only a limited selection of plastics, such as PLA, ABS, PETG, and nylon, is widely suitable for 3D printing.
- Some plastics release harmful fumes during printing.
- Non-biodegradable plastics can negatively impact the environment, especially when used for short-term or single-use prints.
Why Is Polymer Better Than Plastic?
Polymers can be considered better than conventional plastics in some contexts because many natural examples are more flexible, sustainable, and biocompatible. For instance, polymers derived from natural sources like lignin, cellulose, silk, and cotton are often used in clothing, textiles, coatings, and adhesives due to their flexibility. Because these materials are naturally occurring, they break down more quickly in nature than synthetic plastics. Additionally, some polymers, like polyurethane and polyethylene glycol, are used in drug delivery systems and medical implants due to their biocompatibility. Plastics are not without their benefits, however. Ultimately, the determination of which is better, polymer vs. plastic, comes down to the application and volume of parts needed.
Frequently Asked Questions About Polymers and Plastic
Are Polymers Stronger Than Plastic?
No, polymers are not inherently stronger than plastics, and strength depends on the specific material. Some engineered plastics, like nylon or polycarbonate, can be much stronger than natural polymers.
Are Polymers Considered Plastic?
No, only some polymers are considered plastics, typically synthetic ones such as thermoplastics and thermosets. Naturally occurring polymers like cellulose, proteins, and cotton are not plastics.
Are All Polymers Plastics?
No, not all polymers are plastics. However, all plastics are polymers because they are made of repeating molecular chains.
What Polymers Are Not Plastic?
Polymers that are not processed into plastic materials are not considered plastics. Examples include wool, silk, natural rubber, cellulose (in wood and paper), and proteins.
What Is the Difference Between Polymers and Plastics in Terms of Tensile Strength?
Tensile strength in polymers and plastics depends on molecular weight, crystallinity, crosslinking, and processing. Semi-crystalline plastics (like nylon) often have higher tensile strength than amorphous polymers because crystalline regions restrict chain movement. To learn more, see our full guide on Tensile Strength.
What Is the Difference Between Polymers and Plastics in Terms of Hardness?
Hardness is a material’s ability to resist localized deformation. Hardness varies widely across polymers and plastics. Many plastics are engineered for hardness and resistance to localized deformation, especially thermosets and crosslinked types, while elastomeric plastics can be very soft. For more information, see our guide on Hardness.
What Is the Difference Between Polymers and Plastics in Terms of Impact Strength?
Impact strength is a material’s ability to resist fracture due to a sudden applied load or shock. Impact strength depends on polymer type, molecular structure, and crystallinity. Some semi-crystalline plastics (like polyethylene) can have higher impact strength than brittle amorphous polymers, but this is not universal.
Summary
This article presented polymers vs. plastic, explained what each is, and discussed the various differences between the two of them. To learn more about polymers vs. plastic, contact a Xometry representative.
Xometry provides a wide range of manufacturing capabilities and other value-added services for all of your prototyping and production needs. Visit our website to learn more or to request a free, no-obligation quote.
Disclaimer
The content appearing on this webpage is for informational purposes only. Xometry makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through Xometry’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please refer to our terms and conditions for more information.


