Silicones are an exciting group of polymeric materials available in a number of different forms. Their properties give them a nearly limitless potential for tailoring to the exacting requirements of the medical and aerospace industries. They can seal, lubricate, and satisfy a wide range of other applications.
This article will discuss the physical and mechanical properties that make silicone so useful. It will also introduce the four main groups of silicone materials, touch on manufacturing methods, and explain why silicone could be the perfect material for your application. Table 1 summarizes seven key properties of silicone and offers a brief description of each:
| Properties of Silicone | Description |
|---|---|
Properties of Silicone Chemical Reactivity | Description Generally non-reactive |
Properties of Silicone Thermal Conductivity | Description Naturally low thermal conductivity; can be increased as needed by adjusting formulation |
Properties of Silicone Toxicity | Description Non-toxic when used appropriately, with FDA-approved medical and food grades |
Properties of Silicone Water Repellency | Description Highly water repellent |
Properties of Silicone Sealing Ability | Description Silicone can form watertight seals, but does not strongly adhere to many surfaces unless adhesives or primers are used |
Properties of Silicone Resistance to Oxygen, Ozone, and Ultraviolet Light | Description Extremely resistant to degradation by both radiation and oxidation |
Properties of Silicone Electrical Conductivity | Description Silicone is naturally an excellent insulator, but it can be made conductive with the addition of fillers such as carbon black, silver, or other conductive particles |
Properties of Silicone Gas Permeability | Description Silicone is relatively permeable to gases compared to many other polymers, which is why it is used in breathable membranes and medical applications |
Properties of Silicone Thermal Stability | Description Physical and mechanical properties remain stable across a wide temperature range |
Properties of Silicone Organic Solvent Resistance | Description Silicone resists many chemicals but can be degraded by some organic solvents and concentrated acids such as sulfuric or hydrofluoric acid |
Table 1. Properties of Silicone
1. Silicones Are Non-Chemically Reactive and Have Low Thermal Conductivity
It takes a lot of energy to break the bonds of the silicon-oxygen chains that form the polymeric skeleton of silicone molecules. Because most chemicals that silicones come into contact with do not have enough energy to overcome the silicone molecule’s resistance to change, there is little driving force for chemical reactions. Thus, silicone is considered generally non-chemically reactive. These stable silicone bonds are the basis for many desirable properties of silicone, including its non-reactivity in most environments.
Silicones generally have low thermal conductivity. This is because the molecular structure of silicone tends to impede the transfer of heat vibrations from one molecule to the next. This can be desirable for some uses of silicone, like oven mitts. But in other situations, the inability to efficiently transfer heat is a problem. In that case, thermally conductive fillers can be added to the silicone formulation to improve the heat transfer required for the intended application.
2. Silicone Toxicity Is Also Low
Silicone is considered safe for human health, which is why medical- and food-grade silicones are widely used for implants and food-contact products. Food-grade and medical-grade silicone compounds are FDA-approved for use in food-contact applications, and some medical-grade silicones are approved for long-term implantation in the human body. As with all chemicals, however, it is important to use silicone products according to the manufacturer’s instructions to maintain the highest levels of safety.
3. Silicone Has the Ability To Repel Water and Form Watertight Seals
Silicone is what is known as a “hydrophobic” material. It repels water. This is because the methyl groups attached to the silicon-oxygen polymer chain are nonpolar in nature. It is not attracted to water molecules. Its low surface energy gives water molecules no way to spread over and penetrate the silicone surface. Instead, water beads up and runs off. This water repellency, combined with silicone’s ability to bond when formulated as a sealant, enables durable, watertight seals.
4. Silicone Has a High Resistance to Oxygen, Ozone, and UV Light
The silicon-oxygen bonds in silicones are more stable than those between the carbon atoms in organic polymer chains. The amount of energy that can be provided by UV light is higher than that needed to break down C-C bonds, but not enough to damage Si-O bonds. This is why silicones are more resistant to UV light and oxidation than carbon-based plastics. Silicones can withstand harsh outdoor environments, resisting moisture, fuels, oils, UV light, and salt spray.
5. Silicone Is Electrically Insulative As Well as Conductive
Standard silicone rubber is naturally an excellent insulator with high dielectric strength. This makes it ideal for many applications, especially in the medical industry, where insulation is critical. But silicone can be coaxed into being conductive enough for applications like gaskets and static shields. This is accomplished by adding fillers to the silicone, such as carbon, silver, or other conductive materials.
6. Silicone Has Excellent Gas Permeability and Thermal Stability
Silicone’s molecular structure makes it relatively permeable to gases, which allows diffusion of small molecules such as oxygen or carbon dioxide, while remaining water-repellent. This combination of water repellency and gas permeability yields coatings that give us the luxury of water-resistant, breathable fabrics.
A key property of silicone rubbers is their thermal stability. Silicone maintains its mechanical and physical properties across a wide temperature range. Depending on the formulation, silicones can remain stable from about -100 °C up to 250-300 °C, with specialty grades handling even wider ranges.
7. Silicone Has Superior Resistance to Organic Compound Solvents
Silicone is resistant to attack by most chemicals due to its non-reactive structure and low surface energy. However, a few inorganic chemicals, notably sulfuric and hydrofluoric acids at high concentrations, will damage silicones. Organic solvents, such as toluene, mineral spirits, gasoline, and carbon tetrachloride, can cause swelling or deterioration of silicones, with the severity of the effect depending on the concentration and exposure time.
Why You Should Use Silicone as a Material
The properties of silicone offer numerous benefits. Here are a few ways that both manufacturers and end-users can benefit from its unique characteristics:
- Silicones are generally considered safe and are widely used in medical devices, including some implantable applications.
- Silicones maintain dimensional stability, retaining strength and shape across a wide temperature range.
- Silicones are chemically non-reactive and resist degradation from moisture, fuels, oils, heat, cold, salt spray, and ultraviolet light.
- Silicones are highly versatile: they can be supplied as liquids, pastes, rubbery sheets, or rigid plastics, and processed into coatings, molded parts, tubing, or lubricants.
When designing municipal water treatment reactors, I’ve specified silicone in both rubber gaskets and sealants. Certified grades are approved for potable water use, and their durability under demanding conditions makes silicone one of the most dependable materials for long-term reactor performance.
Where is Silicone Typically Prepared?
Chemical factories produce raw silicone materials. These facilities can process the required chemical precursors and catalysts in bulk, repeatedly, and precisely.
In the factory, silica (usually from sand) is reduced to elemental silicon in a high-temperature reaction with carbon. The silicon is then reacted with methyl chloride to form intermediates such as dimethyldichlorosilane, which are hydrolyzed and polymerized to produce basic silicone products in the form of liquids, gels, or sheets. Component fabricators then apply the appropriate molding or other industrial processes to create final products for customers.
What is Silicone's Chemical Composition?
Silicone, technically referred to as “polysiloxane,” consists of a chain of alternating silicon and oxygen atoms. Silicon atoms, like carbon atoms, have space for four covalent bonds with other atoms. Two of those spots are occupied by oxygen atoms, one on each side. The other two spots will bond with other molecular groups that are available. The general chemical formula for silicone is [R2SiO]n. “R” indicates whatever groups are attached to the silicon atoms. The most common silicone is polydimethylsiloxane (PDMS), in which methyl groups (CH3) are attached to the silicon atoms along the Si–O backbone. Its general formula is [–Si(CH3)2–O–]n. Although it contains organic groups, the backbone itself is inorganic (Si–O), giving it hybrid organic–inorganic polymer properties. Figure 1 shows the chemical structure of polydimethylsiloxane.
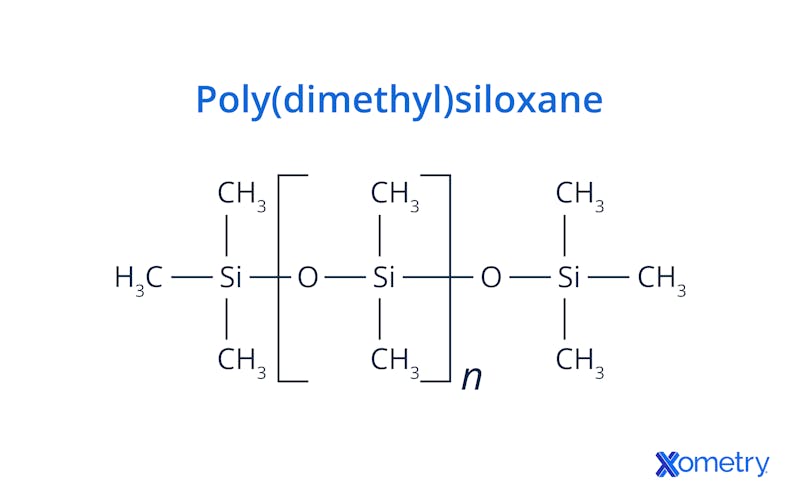
What Are Silicone's Mechanical Properties?
Silicone rubbers are generally strong and flexible over a broad range of temperatures. By design, the mechanical properties of silicone materials cover a wide range. Table 2 below provides a range of results for a variety of mechanical properties of silicone.
Table 2. Typical ranges of mechanical properties for silicone rubber.
| Mechanical Property | Range of Results |
|---|---|
Mechanical Property Shore A Hardness (Resistance to indentation) | Range of Results 10-90 |
Mechanical Property Yield Strength (MPa) (Stress at onset of plastic deformation) | Range of Results 0.05-10 |
Mechanical Property Tensile Strength (MPa) (Stress at failure) | Range of Results 2-12 |
Mechanical Property Elongation at Yield (%) (Sample deformation at yield point) | Range of Results 100-900 |
Mechanical Property Elongation at Break (%) (Sample deformation at tensile failure) | Range of Results 5.00–1490 |
Mechanical Property Modulus of Elasticity (GPa) (Measure of material stiffness in tension) | Range of Results 0.5-15 |
Mechanical Property Compressive Modulus (GPa) (Measure of material stiffness in compression) | Range of Results 5-25 |
Mechanical Property Poisson’s Ratio (Ratio of transverse to axial strain during tensile elongation) | Range of Results 0.500 |
Mechanical Property Compression Set (%) (Permanent deformation after compressive force is removed) | Range of Results 5-50 |
Mechanical Property Tensile Set (%) (Permanent deformation after tensile force is removed) | Range of Results 1.00-10.0 |
Mechanical Property Tear Strength (kN/m) (Force applied for crack propagation) | Range of Results 2-50 |
What Are the Various Types of Silicone?
The four main physical types or forms of silicone products are listed below:
- Room-Temperature Vulcanizing (RTV): These silicones cure at room temperature. They are typically used for sealing and bonding. RTV-1 silicones cure upon exposure to ambient moisture and are typically supplied as one-part sealants. RTV-2 silicones are two-component systems that cure when the base and curing agent are mixed, allowing more controlled processing for molding, potting, and coating. This makes RTV-2 more flexible for applications like molding and coating.
- Liquid Silicone Rubber (LSR): LSR is a two-component, pumpable liquid system that cures rapidly at high temperature, typically using a platinum catalyst, and is processed by injection molding. Both the molding process used and the final product characteristics of LSR are adaptable to a wider range of applications.
- Fluorosilicone Rubber (FSR): FSR molecules consist of repeating units of a silicon-oxygen backbone with other bonding sites occupied by other groups of molecules (methyl and fluoroalkyl groups). This gives the material excellent resistance to fuels, oils, and solvents, making FSR especially useful for sealing applications in aerospace and automotive industries.
- High-Consistency Rubber (HCR): HCR, also known as “heat-curing rubber” or “high-consistency rubber,” is based on very high molecular weight silicone polymers. It is supplied as an uncured, dough-like compound that can incorporate fillers and additives, and is typically processed by compression or transfer molding before heat curing. It is ideal for use in certain medical devices, including tubing, balloons, and sheets.
Silicones can take a variety of forms, from liquid to solid. There are many variations of each type, with the properties of the silicones tailored to the needs of specific end products.
For more information, see our guide on the types of silicone.
Frequently Asked Questions About Silicone Properties
Why is Silicon Classified as a Metalloid?
Silicon is classified as a metalloid because it exhibits both metallic and non-metallic properties. Pure silicon has a shiny, metallic-like appearance but is brittle and behaves chemically more like a non-metal.
However, silicon’s other characteristics are somewhere in the middle between those of true metals, like iron, and true non-metals, like phosphorus. Despite its metallic appearance, silicon is brittle, has relatively low electrical conductivity at room temperature, and shows chemical behavior closer to non-metals.
Summary
This article discussed 7 key properties of silicone, presented the chemical and physical characteristics of the material, and showed the different common forms or types that are used in industry.
Xometry provides a wide range of manufacturing capabilities and value-added services for all of your prototyping and production needs. Visit our website to learn more or to request a free, no-obligation quote.
Disclaimer
The content appearing on this webpage is for informational purposes only. Xometry makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through Xometry’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please refer to our terms and conditions for more information.


