Transition metals, also known as transition elements, are chemical elements found in Groups 3 through 12 of the periodic table. These elements occupy the d-block because their electron configurations involve the progressive filling of d-orbitals. A key feature of transition metals is that they have partially filled d-orbitals, either in their ground state or at least one of their oxidation states. This property gives rise to their unique chemical and physical behaviors, including the formation of colored compounds, multiple oxidation states, and strong metallic bonding.
This article will discuss the various types of transition metals and examine their properties and applications, among other aspects.
What Are Transition Metals?
Transition metals are a group of elements primarily located in Groups 3 through 12 of the periodic table, commonly referred to as the d-block elements. These elements are characterized by the presence of partially filled d-orbitals, either in their neutral atomic state or in at least one of their common oxidation states. This defining feature imparts a wide range of physical and chemical properties, including the ability to form variable oxidation states, complex ions, and colored compounds, as well as catalytic behavior.
According to the International Union of Pure and Applied Chemistry (IUPAC), a transition metal is explicitly defined as an element that forms at least one ion with a partially filled d-subshell. Under this strict definition, zinc (Zn), cadmium (Cd), and mercury (Hg)—which possess filled d-orbitals in both their neutral and common ionic forms—are not considered "true" transition metals, even though they are located within the d-block. Nonetheless, for practical and pedagogical reasons, these elements are often included in general discussions about transition metals.
It is also important to distinguish inner transition metals, which consist of the f-block elements—the lanthanides and actinides—found below the main body of the periodic table. These are sometimes grouped under the broader umbrella of transition metals, but strictly speaking, they are classified separately due to their f-orbital electron configurations. While there are 40 elements in the d-block, only 38 of these elements are considered to be transition metals under conventional periodic table layouts. Lanthanum (La) and Actinium (Ac), though located in Group 3, are more often placed with the inner transition metals (f-block) due to their electron configurations and chemical behavior.
What Are the Different Properties of Transition Metals?
Transition metals show typical metallic behavior. The general properties of transition metals are as follows:
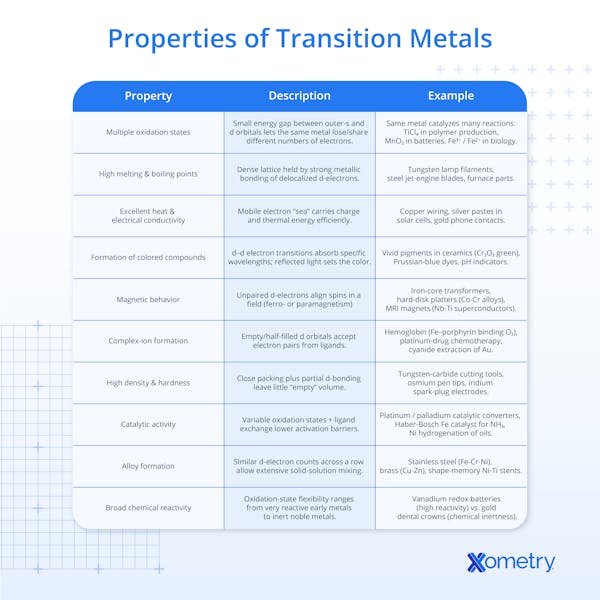
1. Multiple Oxidation States
Transition metals are known for exhibiting a variety of oxidation states in their compounds. This is due to the relatively low energy difference between their outer s orbitals and the d orbitals where the valence electrons reside. As electrons can be added to or removed from both the s and d orbitals, transition metals can participate in a range of redox reactions, enabling them to act as versatile catalysts and reactants. For instance, early in the transition series, metals like titanium can lose electrons from both their 3d and 4s orbitals, leading to a range of stable oxidation states. The variability of oxidation states is further influenced by the metal's position in the periodic table, with elements in the middle of the series typically displaying a wider range of states.
2. High Melting and Boiling Points
Most transition metals have high melting and boiling points. This is attributed to the strong metallic bonds formed by the delocalized d electrons. These electrons move freely throughout the metal lattice, creating a strong bond between the positively charged metal ions. The dense electron cloud resulting from the overlap of d orbitals contributes to the strength of these metallic bonds.
3. Good Conductivity of Heat and Electricity
The free movement of electrons in the electron cloud of transition metals facilitates the efficient conduction of heat and electricity. This property makes them ideal materials for electrical wiring and components that must efficiently dissipate heat.
4. Formation of Colored Compounds
The d-d electron transitions within transition metal compounds are responsible for their vivid colors. When light hits these compounds, electrons are excited from one d orbital to another, absorbing specific wavelengths of light. The wavelengths that are not absorbed are reflected, giving the compounds their characteristic colors. This property is utilized in dyes, pigments, and indicators.
5. Magnetic Behavior
Certain transition metals (e.g., iron, cobalt, and nickel) and their compounds can exhibit paramagnetism when they have unpaired d electrons. The spin of these unpaired electrons can align under the influence of an external magnetic field, making the material magnetic.
6. Formation of Complex Ions With Ligands
Transition metals can form complex ions by coordinating with ligands — molecules or ions that donate pairs of electrons to the metal. This ability to form coordination complexes is fundamental to many biological processes (e.g., oxygen transport by hemoglobin) and industrial applications (e.g., catalysis and the purification of metals).
7. High Density and Hardness
The closely packed crystal lattice and the presence of d electrons contribute to the high density and hardness of transition metals compared to s-block elements. This makes them suitable for structural applications and tools.
8. Catalysts in Chemical Reactions
Transition metals and their compounds serve as excellent catalysts due to their ability to adopt multiple oxidation states and form complexes. They can facilitate chemical reactions without being consumed in the process, thereby significantly increasing the reaction rate. Catalysts like platinum and palladium, for example, are crucial in automotive catalytic converters for the reduction of harmful emissions.
9. Ability To Form Alloys
Transition metals can easily mix with other metals to form alloys, materials with enhanced properties, such as greater strength, corrosion resistance, or electrical conductivity. Steel, brass, and bronze are some examples of popular alloys.
10. Wide Range of Chemical Reactivity
Due to their varied oxidation states and the formation of complexes, transition metals exhibit a broad spectrum of chemical reactivity, from highly reactive metals like scandium and titanium to relatively inert metals like gold, silver, and platinum, often referred to as noble metals. Noble metals exhibit low reactivity due to their high ionization energies and low hydration enthalpies.
Do Transition Metals Have Unique Melting and Boiling Points?
Yes, transition metals are known for their unique and generally high melting and boiling points compared to other elements, particularly those in the s-block of the periodic table. This is explained by their strong metallic bonds, formed by the delocalization of d electrons. These electrons contribute to a cohesive force that holds the metal atoms together tightly, necessitating more energy to break these bonds and change the phase of the metal.
What Is the Use of Transition Metals?
Transition metals are beneficial in several applications due to their unique properties. They serve as catalysts in chemical reactions and are key components in steel and other alloys for enhancing strength and corrosion resistance. They form colored compounds used in pigments and dyes, and are essential in electronic and electrical applications due to their conductivity. They also play critical roles in biological systems, such as oxygen transportation and enzyme reactions.
What Are the Different Types of Transition Metals?
The different types of transition metals are discussed below:
1. Gold (Au)
Gold is an outer transition metal, is a group 11 element, and also forms part of the noble/precious metals. Its atomic number is 79 and its electron configuration is [Xe]4f¹⁴5d¹⁰6s¹. This metal is solid at standard conditions, yellow in color, soft, and considered to be the most malleable and ductile of the elements. It can be made into fragile sheets, known as gold leaf. Gold is one of the least chemically reactive elements of the transition metals. It remains unaffected by oxygen or sulfur but will easily engage in reactions with halogens or with solutions that contain or produce chlorine, such as aqua regia.
Gold is also a good conductor of both heat and electricity and is often used in electronic conductors. However, the most common use of gold is jewelry. One of the major benefits of gold is its corrosion resistance; however, because of its softness and malleability, 24-carat gold (the purest form of gold) is not suited for everyday jewelry. Therefore, gold is often alloyed to perform better in jewelry applications. Lower-grade gold may tarnish depending on the alloying material and composition. Gold can be pretty expensive due to its scarcity and its status as a precious metal.
2. Mercury (Hg)
Mercury is identified with the symbol Hg, atomic number 80, and electronic configuration of [Xe]4f¹⁴5d¹⁰6s². It is an outer transition metal and the only metal in the liquid state at room temperature. It has a shiny, silvery-white appearance with a high surface tension. One of the significant uses of mercury is for measuring pressure. Mercury's high density makes it ideal for use in barometers, allowing for a reasonably sized column to accurately measure atmospheric pressure. In contrast, a water-based barometer would have to be 13.6 times higher than one using mercury to achieve the exact measurement of pressure difference. Mercury is also used in thermometer applications and the manufacturing of fluorescent lamps. However, its significant drawback is its toxicity, posing environmental and health risks. Mercury is also one of the most expensive liquids in the world (about $3,400 per gallon).
3. Silver (Ag)
Silver (Ag) is a group 11 element categorized as an outer transition metal. Its atomic number is 47, with an electron configuration of [Kr]4d¹⁰5s¹. At room temperature, silver is a solid, exhibiting a lustrous white color. It holds the highest electrical and thermal conductivity of all metals, making it ideal for use in electrical components, solar panels, and reflective items. Additionally, its antibacterial qualities find applications in medicine and water purification. Commonly used in jewelry and silverware, silver is alloyed with copper to enhance its durability, due to its natural softness. Despite its reactivity being lower than that of many other metals, silver can tarnish when exposed to ozone, hydrogen sulfide, or air containing sulfur, resulting in a black surface layer of silver sulfide. Silver costs around $25 per ounce, which is significantly cheaper than gold at around $2,200 per ounce.
4. Titanium (Ti)
Titanium (Ti) is an element in group 4 and is recognized as an outer transition metal. With an atomic number of 22, its electron configuration is [Ar]3d²4s². Titanium is a solid at room temperature, characterized by its silver color, high strength, low density, and notable corrosion resistance, particularly against seawater and chlorine. This combination of properties makes it extremely valuable in aerospace, military, and medical applications, such as in aircraft, naval ships, spacecraft, dental implants, and prostheses. Despite its strength, titanium is also highly malleable and ductile when pure. It's one of the few elements that offer a high strength-to-weight ratio, which is crucial in advanced engineering applications. While titanium's reactivity is relatively low, allowing it to resist corrosion, it does react with oxygen at high temperatures, forming a protective oxide layer. This metal is more abundant and less expensive than precious metals like gold and silver, yet it still commands a higher price due to its complex extraction and processing methods.
5. Iron (Fe)
Iron (Fe), a group 8 element, is classified as an outer transition metal with an atomic number of 26. It constitutes about 5% of the Earth's crust, making it one of the most abundant metals. Its electron configuration is [Ar]3d⁶4s², and it has a characteristic lustrous, metallic grayish color. Iron is the most essential metal of all, and the most common use of iron is as a component in steel. This alloy is irreplaceable in the construction, infrastructure, manufacturing, and machinery industries worldwide. It has a high melting point and magnetic properties, but iron starts to corrode when exposed to moist air and higher temperatures, yet remains stable in dry conditions. It easily dissolves in weak acids, demonstrating significant chemical reactivity. Iron is easily alloyed with other elements to improve its strength and resistance to wear and tear.
6. Palladium (Pd)
Palladium (Pd), belonging to group 10 of the periodic table, is an outer transition metal with an atomic number of 46. Its electron configuration is [Kr]4d¹⁰, and it features a silvery-white luster. This metal is renowned for its exceptional ability to absorb hydrogen, making it valuable in catalytic converters for reducing harmful emissions in automobile exhaust. Additionally, palladium plays a crucial role in electronics, fuel cells, dentistry, and jewelry, particularly in white-gold alloys. Its catalytic properties are also exploited in chemical reactions, such as hydrogenation and dehydrogenation processes. Despite its relative scarcity and resulting high cost (around $1,026 per ounce), palladium's unique chemical and physical properties, including excellent corrosion resistance and stability at high temperatures, ensure its continued demand in various high-tech and industrial applications.
7. Copper (Cu)
Copper (Cu) is a group 11 element and an outer transition metal with an atomic number of 29. Its electron configuration is [Ar]3d¹⁰4s¹, and it has a distinctive reddish-brown color. Copper is especially known for its excellent thermal and electrical conductivity. These properties make copper ideal for use in electrical wiring, plumbing, and heating systems. Its antimicrobial properties also find utility in medical and architectural applications. Copper alloys, such as bronze and brass, combine with other metals to enhance strength, durability, and corrosion resistance, thereby broadening their applications in construction, coinage, and the decorative arts. While copper is reactive with atmospheric oxygen, forming a green patina that protects it from further corrosion, it remains chemically active, especially in the presence of acids. Copper is also very affordable compared to precious metals, priced at around $4 per ounce.
8. Cobalt (Co)
Cobalt (Co) is a ferromagnetic element with a silver-white appearance, hardness, and brittleness, along with a shiny luster. It is an outer transition metal that belongs to group 9 of the periodic table with an atomic number of 27 and electron configuration [Ar]3d⁷4s² and, like iron, can become magnetized. Its physical attributes closely mirror those of iron and nickel. Globally, cobalt's primary application is in the electrodes of rechargeable batteries. It is also used in the production of superalloys for gas turbine engine components. Further uses of cobalt include the manufacture of car airbags, cemented carbides and diamond tools for cutting, alloys resistant to corrosion and wear, catalysts for petroleum and chemical processing, drying agents in varnishes, paints, and inks, pigments and dyes, base layers for porcelain enamels, high-speed steel tools, magnets, magnetic recording media, and the steel belts in radial tires. Despite its versatility, cobalt's extraction and processing are challenging, contributing to its relatively high cost ($30,000 per ton).
9. Chromium (Cr)
Chromium (Cr) is a member of group 6 in the periodic table and is an outer transition metal with an atomic number of 24. Its electron configuration is [Ar]3d⁵4s¹, and it has a lustrous, steel-gray appearance. This hard, brittle metal reacts when heated, forming green chromic oxide and showcasing its instability in oxygen by quickly forming a thin, impermeable oxide layer that effectively shields the underlying metal from further oxidation. It is known for its resistance to corrosion and tarnishing when exposed to air. It is extensively used in the plating process to produce a shiny, protective coating for automotive parts, appliances, and furniture. Chromium is also used for hardening steel, creating stainless steel, and forming various alloys. Chromium is quite affordable at around $12,500 per ton.
10. Nickel (Ni)
Nickel (Ni), an outer transition metal from group 10, holds the atomic number 28 with an electron configuration of [Ar]3d⁸4s². This element has a silver-white sheen with a golden tinge that can be polished to a brilliant finish. Known for its strength, ductility, and corrosion resistance, nickel is extensively used in the production of stainless steel and other corrosion-resistant alloys such as nickel-bronze or nickel-silver. The metal's capacity to withstand extreme temperatures and environments makes it essential for the aerospace and engineering fields. Additionally, nickel finds applications in electroplating, providing protective and attractive finishes to a wide variety of products. Given its utility across numerous industries, nickel's value is significant, although it is more accessible in terms of price compared to precious metals.
11. Iridium (Ir)
Iridium (Ir), an outer transition metal, belongs to group 9 of the periodic table. Its atomic number is 77, and it has an electron configuration of [Xe]4f¹⁴5d⁷6s². This element is the most corrosion-resistant metal known to exist. Iridium is extremely hard and has one of the highest melting points among the metals, characteristics that make it exceptionally durable and suitable for high-temperature applications. Due to its stability and corrosion resistance, iridium is utilized in high-temperature environments, such as spark plugs and crucibles for high-temperature apparatus. Furthermore, iridium's high density and ability to absorb considerable amounts of hydrogen make it valuable in the field of catalysis, particularly in the automotive industry for catalytic converters. Due to its scarcity, iridium has a high price point. With an average price of around $4,500 per troy ounce, iridium is more valuable than silver, gold, or platinum
12. Tantalum (Ta)
Tantalum (Ta) is part of group 5 of the periodic table with an atomic number of 73. Its electron configuration is [Xe]4f¹⁴5d³6s². Tantalum, a metal known for its dark blue-gray color, combines density, ductility, and remarkable hardness with ease of fabrication and excellent conductivity of both heat and electricity. It is especially valued for its near-total resistance to corrosion by acids, displaying an exceptional immunity to the usually corrosive aqua regia at temperatures below 150°C. The primary applications of tantalum are found in electrolytic capacitors and chemical equipment that requires corrosion resistance. Tantalum capacitors offer the most significant capacitance per unit volume compared to all other capacitors, making them widely utilized in miniaturized electronic circuits. On the downside, tantalum is quite rare and presents some challenges associated with mining and processing. Tantalum costs around $190 per kilogram of Ta2O5 content.
13. Cadmium (Cd)
Cadmium (Cd), an element in group 12 of the periodic table, has an atomic number of 48 and an electron configuration of [Kr]4d¹⁰5s². This metal is characterized by its soft, bluish-white appearance and is known for its exceptional malleability and ductility. Cadmium is primarily used in the manufacturing of nickel-cadmium (NiCd) rechargeable batteries, leveraging its capacity to withstand electrochemical oxidation and reduction. Additionally, it finds applications in pigments, coatings, and plating to protect against corrosion due to its excellent resistance to alkali metals and low melting point. Cadmium is also utilized in the stabilization of plastics and in some alloys, which require high resistance to fatigue, wear, and deformation. However, due to its toxicity and the environmental concerns associated with its use, cadmium's applications are highly regulated, and safer alternatives are sought in many industries. Cadmium averages at around $3.30 per kilogram.
14. Tungsten (W)
Tungsten (W) belongs to group 6 of the periodic table, has an atomic number of 74, and has an electron configuration of [Xe]4f¹⁴5d⁴6s². Like most metals, it has a grayish-white, lustrous appearance. It is distinguished by its extraordinary hardness and high melting point, the highest of all metals, and the most scratch-resistant of all metals. It was once commonly used in incandescent light-bulb filaments but has been largely phased out due to poor energy efficiency. Now, it’s used in high-temperature applications, such as arc-welding electrodes and furnace elements. Tungsten is also widely used in the production of hard materials like tungsten carbide and in making cutting and drilling tools. Despite its strength, tungsten is very brittle, making it challenging to work with in its pure form. Tungsten is also cheap compared to precious metals like gold and is therefore becoming a popular choice for wedding bands.
15. Rhodium (Rh)
Rhodium, with its placement in group 9 and an atomic number of 45, has an electron configuration of [Kr]4d⁸5s¹. This rare, silver-white metal is highly reflective and known for its excellent corrosion resistance. Rhodium is primarily used in automotive catalytic converters, which reduce harmful emissions by catalyzing the breakdown of nitrogen oxides. Additionally, it is used as a catalyst in the chemical industry for the production of nitric acid and acetic acid, as well as in hydrogenation reactions. It also finds application in jewelry as a plating material due to its reflective surface and ability to resist tarnishing. Furthermore, rhodium is utilized in the chemical industry as a catalyst for various organic reactions. Rhodium is the most expensive metal in the world, with an average cost of over $13,000 per troy ounce. Rhodium's high cost is significantly influenced by its rarity, with an annual production of only about 30 metric tons (in 2022). This is in stark contrast to the larger quantities of other precious metals produced in 2022, such as approximately 210 metric tons of palladium, 190 metric tons of platinum, and 3,100 metric tons of gold.
16. Manganese (Mn)
Manganese (Mn), a group 7 element with an atomic number of 25, has an electron configuration of [Ar]3d⁵4s². It also has a silver-gray color and is both hard and very brittle. Manganese is a highly reactive metal that readily combines with oxygen to form various oxides, like manganese dioxide (MnO2) and manganese heptoxide (Mn2O7). This element has a relatively high electronegativity, which draws electrons towards itself in chemical reactions. Manganese compounds vary in solubility; manganese dioxide is water-insoluble, whereas manganese sulfate dissolves easily. Additionally, manganese can create complex compounds with ligands such as water and ammonia. Manganese is crucial in the steel-making process, where it is added to improve the strength, toughness, workability, and wear resistance of both steel, especially stainless steel, and aluminum alloys. Its compounds are used in the production of batteries, particularly in alkaline and lithium-ion batteries, as well as in various industries as a pigment. Manganese also plays a vital role in biological systems as an essential nutrient for many forms of life. The cost of manganese is around $1,400 per ton. Manganese ore prices, on the other hand, can fluctuate a bit, but they’re more or less in the range of $490 to $520.
17. Vanadium (V)
Vanadium (V), found in group 5 with an atomic number of 23, has an electron configuration of [Ar]3d³4s². This soft, silvery-gray metal is known for its ability to form stable compounds with various oxidation states. Vanadium is primarily used to produce ferrovanadium, an important alloying agent in the production of steel. The addition of vanadium improves the strength, hardness, and resistance to shock and corrosion in steel. It is also used in catalysts for the production of sulfuric acid and in aerospace applications, in which strong, corrosion-resistant materials are required. Vanadium's role in energy storage is emerging, particularly in the development of vanadium redox flow batteries for large-scale energy storage systems. However, the energy density is not as high as that of lithium-ion batteries. While vanadium might seem rare, its presence in the Earth's crust is comparable to metals like copper, nickel, and zinc. However, extracting vanadium is costly, with ore typically containing about 1.5% vanadium pentoxide, making it one of the more expensive elements to recover from the earth.
18. Zirconium (Zr)
Zirconium, symbol Zr, atomic number 40, and electron configuration of [Kr]4d²5s², is crucial in the nuclear industry due to its low neutron absorption. This strong transition metal is renowned for its ability to form a wide range of organometallic and inorganic compounds. It boasts exceptional resistance to both corrosion and high temperatures. Its hardness is comparable to that of copper, yet it has a lighter weight than steel. Zirconium has similar physical and chemical properties to titanium. Zirconium is widely used in industries ranging from aerospace, where it's a component in jet engines and space shuttles, to medical applications, serving as a biocompatible material for implants. Its corrosion-resistant properties also make it valuable in creating alloys and ceramics, as well as in applications such as catalytic converters. The average import price for zirconium in the United States is approximately $30 per kilogram.
19. Osmium (Os)
Osmium (Os) is the densest naturally occurring element, with a density of 22.5 g/cc. It is also a brittle transition metal in group 8 of the periodic table, with an atomic number of 76. Its electron configuration is [Xe]4f¹⁴5d⁶6s². Osmium has a distinctive blue-white metallic luster and is very brittle, hard, and challenging to work with, even at high temperatures. Among the platinum group metals, osmium possesses the highest melting point, making melting and casting operations challenging. Before tungsten became the material of choice, osmium wires were employed as the filament in the initial versions of incandescent light bulbs. Despite its impressive attributes, osmium is rarely used in bulk due to its rarity and the toxic nature of its oxide when exposed to air, limiting its applications to specialized fields. The cost of osmium is around $400 per ounce.
20. Platinum (Pt)
Platinum (Pt), with an atomic number of 78, is a precious metal in group 10 of the periodic table. Its electron configuration is [Xe]4f¹⁴5d⁹6s¹. This transition metal is very dense, malleable, and ductile (more so than gold, copper, or silver), and it also exhibits very high corrosion resistance. Platinum is unaffected by both air and oxygen, but it will dissolve in aqua regia. The many uses of platinum include jewelry, surgical tools, electrical wires, laboratory equipment, the glass industry, catalysts in chemical processes (such as the refining of petroleum and the production of nitric acid, silicone, and benzene), catalytic converters for reducing vehicle emissions, and in fuel-cell technologies. Platinum costs around $913.50 per troy ounce.
21. Molybdenum (Mo)
Molybdenum (Mo), with the atomic number 42 and positioned in group 6 of the periodic table, has an electron configuration of [Kr]4d⁵5s¹. It has a dark gray color. This transition metal is very hard, but softer and more ductile than tungsten. As an alloying agent, molybdenum is used to improve the hardenability, toughness, and high-temperature strength of steels. It is also used as an alloying element in nickel-based alloys like Hastelloys®. Its applications range from serving as electrodes in glass furnaces and components in nuclear energy to being a crucial catalyst in petroleum refining, as well as being used in missile and aircraft parts. Additionally, molybdenum is a key material in the electronic and electrical fields, used in filament manufacturing and circuit inks due to its conductivity. The primary drawback of molybdenum is its limited resistance to oxidation at high temperatures, with rapid oxidation occurring above 600 °C, and its low ductility at room temperature. Molybdenum prices average at around $48,300 per metric ton.
22. Niobium (Nb)
Niobium has the symbol Nb, atomic number 41, and an electron configuration of [Kr]4d⁴5s¹. This element is recognized for its superconducting properties. Niobium enhances the strength of alloys, especially at low temperatures, and is incorporated into materials such as stainless steel. Its addition is crucial for the manufacture of jet engines and rockets, as well as for beams and girders used in building structures and oil rigs. Niobium alloys also play a significant role in the construction of oil and gas pipelines. At present, the cost of niobium is approximately $45 per kilogram (or $45,000 per ton) for standard ferroniobium metal, while niobium pentoxide (Nb2O5) exceeds $50 per kilogram. Products of higher purity will be more expensive.
23. Yttrium (Y)
Yttrium (Y), a silver-metallic transition metal with the atomic number 39, is a member of the rare earth elements. It has an electron configuration of [Kr]4d15s2. This element is not found free but occurs in small amounts in a variety of minerals, such as xenotime and monazite. Yttrium is known to improve the strength and workability of aluminum and magnesium alloys, making these materials more durable and resistant to high temperatures. Additionally, it finds application in creating microwave filters for radar systems and acts as a catalyst in the polymerization of ethene. One of the most significant uses of yttrium is in the production of yttrium-aluminum-garnet (YAG) lasers, which are capable of cutting through metals. Yttrium metal, with a purity of 99.9%, is around $30 per kilogram.
24. Technetium (Tc)
Technetium (Tc) is a synthetic element with atomic number 43 and electron configuration [Kr]4d55s2. It was the first element to be artificially produced. It resembles platinum but is typically in the form of a gray powder. It is considered to be a semiconductor below 11.2 K (-261.95 °C). Technetium primarily exists in the form of radioactive isotopes, with technetium-99m (211,000-year half-life) being the most utilized in medical diagnostic procedures, especially in nuclear medicine for its ability to emit gamma rays without heavy radiation doses. Its radioactivity, however, poses challenges for storage and disposal. Another use of technetium is as a corrosion inhibitor for steel.
25. Scandium (Sc)
This is a rare-earth element with the symbol Sc, atomic number 21, and electron configuration [Ar]4s²3d¹. It's known for its lightweight and high melting point, making it valuable in aerospace and high-performance materials. Scandium enhances the properties of aluminum alloys, increasing their strength and resistance to corrosion, which is why it's utilized in aerospace components, sports equipment like bicycle frames, and even in some baseball bats. Despite its usefulness, scandium's rarity and the difficulty of its extraction contribute to its high cost (approximately $3,500 per kilogram) and limited application.
26. Rhenium (Re)
Rhenium has the symbol Re, atomic number 75, and electron configuration [Xe]4f145d56s2. Rhenium is a rare, silvery-white metal known for its exceptional hardness and resistance to wear and corrosion. With a melting point of 3,180 °C, it boasts one of the highest melting points among all elements, surpassed only by tungsten and carbon. Rhenium is utilized in various applications, including as an additive in tungsten- and molybdenum-based alloys, in filaments for mass spectrographs and photoflash lamps, and as a material for electrical contacts due to its excellent wear resistance and ability to withstand arc corrosion. It also plays a role in catalysts and thermistors. The current price for this metal is around $2,000 per kg.
27. Hafnium (Hf)
Hafnium (Hf), atomic number 72, belongs to group 4 of the periodic table, distinguished by its [Xe]4f¹⁴5d²6s² electron configuration. It is a lustrous, silver-colored metal that exhibits excellent corrosion resistance and can be easily drawn into wires. Known for its impressive neutron absorption capability, it is used in the manufacture of control rods for nuclear submarines. Additionally, its exceptionally high melting point makes it a valuable component in plasma welding torches. It is also used in the semiconductor industry. All hafnium compounds should, however, be considered toxic. The price of hafnium has increased significantly in recent years (from $1,632 per kg in 2022 to $4,457 per kg at the beginning of 2024); this is attributed to its increase in demand.
28. Ruthenium (Ru)
Ruthenium (Ru) is a transition metal with an atomic number of 44 and an electron configuration of [Kr]4d75s1. It is a hard, brittle, silver-gray metal with a high resistance to corrosion and a high melting point. It is one of the rarest elements found in the Earth’s crust, which makes it fairly expensive. Ruthenium is mostly used in microelectronics, as well as a hardener for platinum, and as a catalyst in the production of certain advanced materials. Ruthenium costs around $15.88 per gram as of March 2024.
29. Rutherfordium (Rf)
Rutherfordium (Rf) is a radioactive synthetic element with an atomic number of 104 and an electron configuration of [Rn]5f146d27s2. Due to its short half-life and extremely limited production, Rutherfordium has no known commercial or industrial applications and is used exclusively in scientific research, particularly in the study of transactinide elements and relativistic effects on heavy atoms. Its physical and chemical properties have not been directly measured; instead, most knowledge about Rutherfordium is based on theoretical predictions and comparisons with lighter Group 4 elements.
30. Seaborgium (Sg)
Seaborgium (Sg) is a synthetic element with an atomic number of 106 and an electron configuration of [Rn]5f146d47s2. It exhibits radioactivity, but aside from this, little is known about the metal. It has no known applications outside of research, and its properties are predicted to be similar to those of tungsten.
31. Roentgenium (Rg)
Roentgenium (Rg) is a radioactive synthetic element with an atomic number of 111 and an electron configuration of [Rn]5f146d107s1. It is used only for research purposes and to synthesize isotopes of other elements. It has a metallic gold appearance, but its chemical properties have not been measured due to its short half-life of approximately 22.6 seconds.
32. Copernicium (Cn)
Copernicium (Cn) is a highly radioactive synthetic element with an atomic number of 112 and an electron configuration of [Rn]5f146d107s2. Its most stable isotope has a half-life of around 34 seconds. With such a short half-life, it has no known uses, and its properties have not been measured.
33. Meitnerium (Mt)
Meitnerium (Mt) is a radioactive synthetic element with an extremely short half-life of roughly 3.8 to 4.5 milliseconds. This is for the most stable known isotope meitnerium-278. The half-life can differ for different isotopes, and meitnerium-282 may have a longer half-life, potentially reaching 67 seconds. It has an atomic number of 109, with an electron configuration of [Rn]5f146d77s2. It has no known uses outside of research, and none of its properties have been measured, other than its radioactivity.
34. Hassium (Hs)
Hassium (Hs) is a synthetic element that exhibits slight radioactivity. It has an atomic number of 108 and an electron configuration of [Rn]5f146d67s2. Little is known about this transition metal due to its half-life of roughly 9.7 seconds. It has no known uses, but is expected to be metallic gray or silvery white, with physical properties similar to those of osmium.
35. Dubnium (Db)
Dubnium (Db) is a radioactive synthetic element. It has an atomic number of 105 and an electron configuration of [Rn]5f146d37s2. The most stable isotope of dubnium has a half-life of about 32 hours, making it unsuitable for any practical use. As such, little is known about its physical properties, and it has no known uses.
36. Darmstadtium (Ds)
Darmstadtium (Ds) is a radioactive synthetic element with an atomic number of 110 and an electron configuration of [Rn]5f146d87s2. It has an extremely short half-life, with 20 seconds being the longest half-life of any of its isotopes. As such, the only property of darmstadtium that has been measured is that it is radioactive. It has no other measured properties and no known uses.
37. Bohrium (Bh)
Bohrium (Bh) is another radioactive, synthetic element. It has an atomic number of 107, with an electron configuration of [Rn]5f146d57s2. Its only known use is for research purposes, which include studying the physical properties of bohrium itself, as well as synthesizing isotopes of other elements.
38. Zinc (Zn)
Zinc, or Zn, is a naturally occurring transition metal with an electron configuration of [Ar]3d104s2, and an atomic number of 30. Zinc is bluish-silver, but it oxidizes to form a protective film. Although Zinc, along with cadmium and mercury, are technically not considered to be typical transition metals (according to the definition) due to their filled d-orbitals, they still form part of the transition metals in the periodic table. This metal is brittle at ambient temperatures. Zinc is primarily used to galvanize metals such as iron to protect against oxidation. It is also used to form alloys such as brass and nickel silver, as well as in the manufacturing of a variety of products such as paints, batteries, inks, and cosmetics. Zinc costs around $2,495 per metric ton as of September 2023.
How To Choose Which Type of Transition Metals To Use
Choosing the appropriate type of transition metal depends on the specific requirements of the application, such as strength and durability, corrosion resistance, electrical and thermal conductivity, catalytic properties, temperature resistance, and biocompatibility. The selection is based on matching the metal's properties — such as melting point, reactivity, strength, and conductivity — to the application's specific needs.
What Type of Transition Metals Can Be Used in Embossing?
Some transition metals used in embossing include zinc, copper, steel (an alloy containing iron), and brass (an alloy of copper and zinc).
What Type of Transition Metals Can Be Used in Laser Engraving?
Most transition metals can be used for laser engraving. Some transition metals commonly used in laser engraving include gold, silver, titanium, iron, copper, cobalt, and platinum.
To learn more, see our complete guide on What is a Laser Engraver.
What Is the Advantage of Using Transition Metals?
Transition metals share several key advantages that make them valuable in industrial, chemical, and electronic applications:
- They are good conductors of electricity and include essential metals used in electronics such as copper, silver, and gold.
- They function as effective catalysts in both industrial and laboratory chemical reactions. Examples include iron in the Haber process and platinum in catalytic converters.
- They are renowned for their exceptional mechanical strength, toughness, and durability, making them ideal for structural and high-stress applications.
- They typically exhibit high melting points and excellent thermal stability, enabling them to perform well under extreme heat conditions.
- Transition metals within the same period (row) of the periodic table can often form solid-solution alloys, making them highly adaptable for specialized alloy development.
What Are the Disadvantages of Using Transition Metals?
While transition metals offer numerous advantages, their use also presents several drawbacks, particularly when compared to alternative materials such as composites or advanced polymers:
- They tend to be dense and heavy, which can be a limitation in applications where weight reduction is critical (e.g., aerospace, automotive, and portable electronics).
- Some transition metals are challenging to process due to their hardness, high melting points, or chemical reactivity. This can complicate machining, forming, or refining operations.
- Several key transition metals—such as platinum, rhodium, and rhenium—are extremely rare in the Earth's crust, making them significantly more expensive than more abundant metals. This rarity impacts both availability and cost, particularly for high-tech or catalytic applications.
Common FAQs About Transition Metals
Do Transition Metals Commonly Exhibit High Melting and Boiling Points?
Yes, one property that transition metals have in common is that they typically exhibit high melting and boiling points.
What Distinguishes Transition Metals From Other Types of Metals?
Transition metals differ from other metals primarily in their ability to form compounds in multiple oxidation states, their high melting points and densities, and their role as catalysts in chemical reactions. Additionally, they are stronger, denser, and more complex compared to group 1 alkali metals and possess lower reactivity. Transition metals also exhibit unique properties such as the formation of colored compounds and magnetic behavior.
What Is the Difference Between Inner Transition Metals and Outer Transition Metals?
The main difference between transition metals and inner transition metals lies in the location of their valence electrons; transition metals have valence electrons in the outermost d orbital, while inner transition metals have valence electrons in the penultimate f orbital, which is more internal.
Summary
This article presented transition metals, explained each of them, and discussed them in detail. To learn more about transition metals, contact a Xometry representative.
Xometry provides a wide range of manufacturing capabilities and other value-added services for all of your prototyping and production needs. Visit our website to learn more or to request a free, no-obligation quote.
Copyright and Trademark Notice
- Hastelloy® is a registered trademark of Haynes International, Inc.
Disclaimer
The content appearing on this webpage is for informational purposes only. Xometry makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through Xometry’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please refer to our terms and conditions for more information.


