Aluminum is one of the most loved manufacturing metals because it’s both lightweight and strong, so it can be used for making everything from cookware to car parts. Many manufacturers like to anodize this metal because the process makes it more wear- and corrosion-resistant. If you’d like to learn how the process works and what options are available, keep on reading.
What is Aluminum Anodizing?
When exposed to the air, aluminum will naturally form a very thin oxide layer that builds up and keeps the material protected, but not for long. Anodizing is an electrolytic process that can ramp up this protection by making the oxide layer thicker and with an ordered structure. The new anodized layer is porous, which helps with sealing or coloring the metal with dye. It’s an affordable process, and you don’t need any special skills or equipment to do it.
Anodizing is particularly helpful for products that will get lots of outdoor use and be exposed to the elements. These include parts for bikes, cars, electrical enclosures, and outdoor furniture. The treatment also makes the material scratch-resistant, and it can act as an insulator since the coating is not conductive. That’s another reason it’s used for boats, architectural cladding, canoes, and even kitchen utensils. When aluminum is anodized, its sealed surface makes it easier to clean and maintain as it won’t react with elements that could otherwise stain it.
How it Works
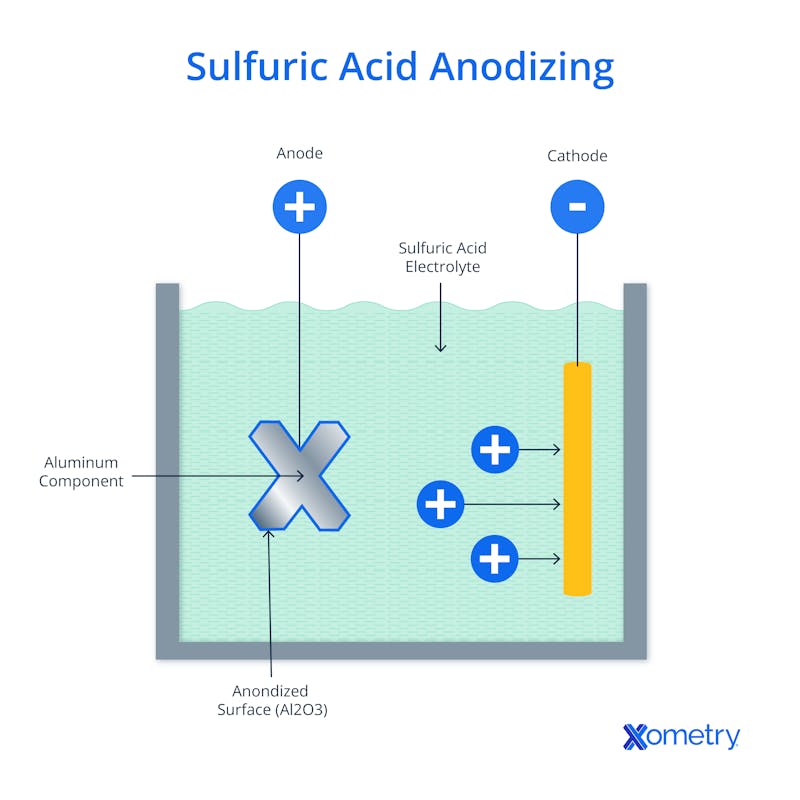
To begin the anodizing process, three things are needed: an anodizing tank, a positive electrode (anode), and a negative electrode (cathode). First, the aluminum needs to be cleaned and its natural oxide layer removed. It’s then dipped into the anodizing tank, which has an electrically conductive solution in it. The solution is zapped with a current to release the aluminum’s electrons, leaving behind positively charged aluminum ions. During the process, the electrolyte completes the circuit between the anode and cathode, which can conduct electricity but won’t react with the electrolyte. Depending on the type of anodizing, that electrolyte will usually be something like sulfuric or chromic acid.
The electrons that leave the cathode are involved in producing negatively charged oxygen ions, which travel to the aluminum’s surface and join with the ions, creating a shiny new thin layer of aluminum oxide. You can adjust the thickness of that layer by controlling the density of the current, the time, temperature, and concentration of the electrolyte solution.
The first oxide layer that’s formed is often referred to as the barrier layer, and it’s continuous without any pores. But as the oxide layer continues to build up, it becomes increasingly porous because it starts restricting the flow of current, and this begins to create attachment points on the barrier layer which develop into small cylindrical pores that are orthogonal to that layer. As that happens, the current starts to spread out from the center of each of those pores, forcing the oxide layer to keep growing until it meets the oxide layers of nearby pores. The following image illustrates this process:
Different Processes
There are three different variations of this process. Here’s what they are:
Chromic Acid Anodizing
The electrolyte in this instance is chromic acid, which makes the thinnest coating of every method—2.5μ, or 0.0001 in. to be precise. Don’t be fooled, though. Although it’s very thin, it makes the aluminum almost as resistant to corrosion as the other methods. The downside of this method, however, is that it’s not as porous and won’t accept color as well as the others do.
Sulfuric Acid Anodizing
As you may have guessed, dilute sulfuric acid is used as the electrolyte in this method, and it’s probably the most often used technique, with a thickness ranging from 5.1 to 30.5μ, or 0.0002 to 0.0012in. An industry standard that we, and most manufacturers, adhere to is:
MIL-A-8625/MIL-PRF-8625 Type II, Class 1 (non-dyed) or Class 2 (dyed)
This method actually produces a harder, more durable coating than chromic acid anodizing does, and it can be colored easily. The downside for some is that the colors can’t always be matched to specific Pantone or RAL colors because of variability in the process. But, compared to chromic acid, sulfuric acid tends to be cheaper, which is another benefit. Here’s an example of a Xometry logo we anodized:

Anodized Aluminum Xometry "X"
Hardcoat Anodizing
This type still uses sulfuric acid as the electrolyte, but it’s designed to make much thicker coatings (usually between 12.7μ and 50.8μ or 0.0005 and 0.002in.) because it uses a higher voltage, longer immersion time, and a lower bath temperature. This coating can even be harder than tool steel, making it great for high-wear situations, and because of its thickness, it tends to darken the aluminum quite a bit. It can still be colored, but it’s harder to do because the pores are smaller and less receptive to dyes.
Frequently Asked Questions on Aluminum Anodizing
What colors are available?
You can dye anodized aluminum in almost any color you like, but as we covered earlier, don’t get your hopes up when trying to match a color perfectly. You should expect a fair amount of color variation with these parts. If you wanted to, you could remove the shine by bead-blasting the part before anodizing it, which will give you a matte finish. When it comes to how to add color, you have two options: electrolytic coloring or dip coloring. The former uses metal salts that bond to the oxide layer, and the latter involves dipping the anodized part into a dye bath. If you want to make a colored part with extra UV resistance, go with electrolytic coloring. Here are some anodized aluminum parts we made at Xometry:

Are there any disadvantages to anodizing aluminum?
Like any process, this one has a few downsides. One of them is that, because of slight differences in composition between pieces of aluminum within the same grade, the surface finish could vary, making it very hard to color-match parts. Remember that although all types of aluminum can be anodized, not all of them react well to it. The 5, 6, and 7xxx series of aluminum alloys are usually the best for anodizing.
What other equipment do I need for the process?
In addition to the components mentioned earlier, you’ll need a DC power source to provide the current, a conductive wire to complete the circuit from the power source to both the anode and cathode, as well as a degreaser, etchant, and dye for coloring the part when you’re done.
How Xometry Can Help
If you want more information on this topic or anything else related to manufacturing, why not reach out to one of our representatives, who would be happy to help? In addition to anodizing, Xometry offers a huge range of related services, including CNC machining, laser cutting, and 3D printing. You can get started right away by requesting a free, no-obligation quote.
Disclaimer
The content appearing on this webpage is for informational purposes only. Xometry makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through Xometry’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please refer to our terms and conditions for more information.


