Ductility is a material property that describes a substance’s ability to undergo inelastic deformation under tensile stress, specifically after the yield point and before fracture. Ductility is measured either by the percentage of elongation or the percent reduction in the cross-sectional area that takes place before a fracture occurs under tensile stress. Highly ductile materials like copper, gold, and aluminum can undergo significant plastic deformation before a fracture happens. Ductility is a key property in the working of metals, and in concert with malleability, it is the basis for much of the shaping and forming of metals that allows complex parts to be made.
This article will examine six of the most ductile metals, how ductility is quantified, the factors that influence it, and typical applications of ductile metals in industry.
1. Gold
Gold is widely recognized as one of the most ductile metals, particularly in its high-purity form. This exceptional ductility allows it to be drawn into extremely fine wires—as thin as a few nanometers—or hammered into ultra-thin sheets, a process known as gold leafing. Unlike brittle materials, gold can undergo this significant deformation without fracturing. Its high ductility stems from its face-centered cubic (FCC) crystal structure, enabling easy dislocation movement. This means that under tensile stress, atoms in the lattice can slide past one another, breaking and reforming metallic bonds without compromising the integrity of the material.
Gold's ductility is more than just a physical curiosity. It is a critical property that makes the metal ideal for high-precision uses, such as fine electrical contacts, microelectronic circuitry, and biocompatible medical implants. In all these cases, gold's ability to maintain structural integrity while being shaped into complex forms is essential to its performance.
2. Platinum
Platinum is often regarded as one of the most ductile metals, rivaling even gold in its ability to undergo extensive plastic deformation without breaking. In controlled conditions, a single gram of platinum can be drawn into a wire several kilometers long, demonstrating its exceptional tensile deformability. Like gold, this high ductility is attributed to its face-centered cubic (FCC) crystal structure, which allows dislocations to move freely and enables atoms to shift positions under stress without causing fracture.
This property makes platinum especially valuable in applications that require fine, durable, and chemically stable components. It is widely used in electrical contacts, where precision and resistance to corrosion are critical, as well as in jewelry, where malleability and luster are prized. Additionally, platinum plays a key role in catalytic converters due to its chemical stability and ability to withstand high temperatures while maintaining its form.
3. Copper
Copper is a highly ductile metal, thanks to its face-centered cubic (FCC) crystal structure, which enables extensive plastic deformation before fracture. Its ductility, combined with excellent electrical and thermal conductivity, makes it ideal for drawing into thin wires without compromising performance or durability. In addition to being ductile, copper is abundant, cost-effective, and easy to refine, contributing to its widespread use in electrical wiring, power transmission systems, and electronic components. Its ability to undergo repeated bending and coiling without breaking is a key reason it remains the industry standard for electrical conductors.
For more information, see our guide on Copper.
4. Nickel
Nickel is a highly ductile metal capable of being drawn into thin wires and rolled into sheets without fracturing. Its ductility is a result of its face-centered cubic (FCC) crystal structure, which facilitates the movement of dislocations and allows atomic bonds to break and reform easily under stress. Beyond its ductility, nickel is valued for its mechanical strength at elevated temperatures, which makes it ideal for high-temperature industrial applications, such as turbine blades, heat exchangers, and components in the aerospace and power generation sectors. Its resistance to corrosion and oxidation also enhances its performance in demanding environments, making it an essential element in superalloys and stainless steels.
5. Niobium
Niobium is a soft, gray transition metal known for its high ductility, which allows it to be drawn into wires or formed into thin sheets without cracking. Unlike most metals commonly noted for ductility, niobium has a body-centered cubic (BCC) crystal structure rather than face-centered cubic. This makes its ductility somewhat unusual, as BCC structures typically offer less slip between atomic planes. However, niobium maintains ductile behavior, particularly at elevated temperatures where atomic movement becomes more favorable. While niobium is not widely used as a structural material on its own, it plays a critical role as an alloying element. When added to steel and stainless steel, it enhances strength, toughness, and corrosion resistance while contributing to overall ductility. This makes niobium-containing alloys suitable for demanding applications, including pipeline steels, jet engines, and superconducting materials.
6. Tantalum
Tantalum is a highly ductile metal capable of being drawn into fine wires or shaped into complex geometries without cracking. Its excellent workability makes it well-suited for manufacturing intricate components and profiles, even under demanding fabrication conditions. Although it has a body-centered cubic (BCC) crystal structure, like niobium, tantalum maintains notable ductility, particularly at elevated temperatures where atomic slip is more favorable. Tantalum is also known for its exceptional corrosion resistance, particularly against acids such as hydrochloric and sulfuric acid. This makes it an ideal material for use in chemical processing equipment, heat exchangers, and reactor components, where both formability and chemical durability are required.
Ductile metals play a key role in modern engineering and manufacturing, enabling the production of fine wires, thin sheets, and intricate shapes that support many technologies. Their ability to deform without breaking is essential not only for shaping processes like drawing and forging but also for enhancing the toughness and reliability of finished components. While gold and platinum often capture attention for their extreme ductility, more common metals like copper and nickel are just as critical in everyday infrastructure and electronics. The continued development of alloys and processing methods aimed at improving ductility underscores its importance as industries seek materials that combine formability, strength, and durability.Mahder Tewolde, Ph.D, PENote from the Editor
What Does Ductile Metal Mean?
If metal is ductile, it can undergo significant deformation before a fracture happens. The technically correct definition of the ductility of a material is either the percentage elongation or the percentage cross-sectional area reduction that a material can withstand while undergoing pure tensile loading.
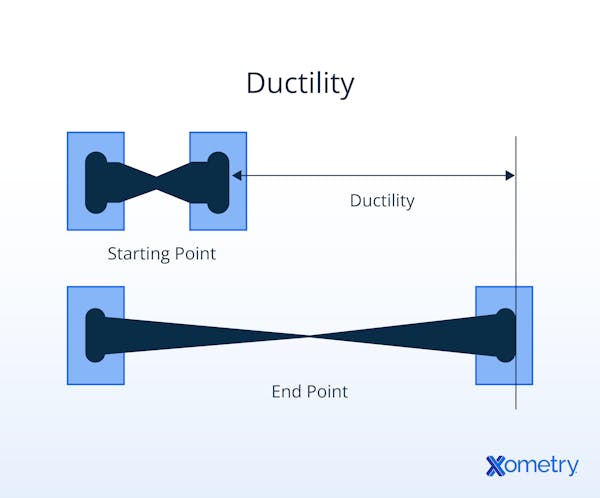
What Makes a Metal Ductile?
Metals that have a simple face-centered cubic crystalline structure and tend to form large regions of crystallization are generally more ductile. Stacked layers of atoms can slide over each other along slip planes joined by the weak atomic (electrostatic) force, where bonds reform as a new lattice position is reached. Where crystal growth regions are smaller, there is less order in the lattice structure, so the atoms slide and reform bonds less well. All materials (brittle and ductile) experience an elastic phase, albeit potentially very short. At the end of the elastic phase, additional load induces intra-atomic movement or yield, when slip planes move against each other. Ductile metals have a limited elastic phase and a long yield phase before fracture.
What Are the Characteristics of a Ductile Metal?
The characteristics of a ductile metal are listed below:
- High elongation under tensile stress, allowing them to be drawn into wires or stretched many times their original length without breaking. This property is commonly measured by percentage elongation in a tensile test.
- The presence of atomic slip systems, particularly in metals with face-centered cubic (FCC) crystal structures, enables layers of atoms to slide past each other easily. This atomic movement is central to ductility.
- Short elastic region and extended plastic region in a stress-strain curve. Ductile metals typically exhibit a relatively limited elastic (reversible) deformation followed by a long plastic (permanent) deformation phase before failure.
- Variable fracture modes, where even ductile materials may ultimately experience brittle fracture under certain conditions, such as low temperatures or high strain rates. However, under standard conditions, they typically show ductile fracture characterized by necking and void formation.
- Improved ductility in engineered forms, such as ductile (nodular) cast iron, which contains spherical graphite nodules instead of flakes. This structure allows for limited plastic deformation, unlike traditional gray cast iron, which is brittle due to its flake graphite structure.
Where Are Ductile Metals Used?
Ductile metals are widely used across manufacturing industries where fine, complex, or highly formed parts must be produced efficiently. Their ability to undergo significant plastic deformation without fracturing makes them ideal for processes such as drawing, rolling, forging, and extrusion. Beyond ease of manufacturing, ductility also contributes to toughness. Materials that can deform plastically under overload—rather than fail abruptly—are more likely to survive extreme or unexpected conditions. In this way, ductile metals help improve the functional reliability of components in structural, automotive, aerospace, and industrial applications.
In contrast, brittle materials are typically reserved for applications where tensile or impact stresses are minimal, such as in compression-only structures or wear-resistant surfaces. When components are subjected to complex loading conditions, including tensile forces, cyclic stresses (fatigue), vibration, or impact, ductile metals consistently offer better mechanical performance and damage tolerance.
What Are the Applications of Ductile Metals?
Listed below are some applications of ductile metals:
- Steel processing often relies on a combination of ductility and malleability. Annealing and hot working are commonly used to enhance ductility during rolling, bending, and forging, particularly in structural and automotive steel production.
- Aluminum is also widely used in forming operations. Its natural ductility at lower temperatures allows for cold rolling and shaping, which promotes work hardening, increasing strength without requiring thermal treatment.
- Wire drawing is a direct application of ductility. Metals such as gold and platinum are drawn cold due to their exceptional ductility, while materials like nickel and stainless steel may require intermediate annealing to maintain formability. Steel wires are often drawn at elevated temperatures to reduce work hardening and increase elongation.
- Solders, typically composed of tin-lead or lead-free alloys, are highly ductile, allowing them to form stress-tolerant joints between less ductile or brittle components in electronics and plumbing.
- Jewelry manufacturing relies heavily on ductile metals like gold, silver, and platinum, often worked cold to create detailed and complex shapes without cracking.
How Is the Tensile Strength of Ductile Metals?
There is no fixed or direct correlation between tensile strength and ductility, as these are distinct material properties. Tensile strength, typically defined as the maximum stress a material can withstand before fracture, is often referred to as ultimate tensile strength (UTS). While ductile materials can sustain large amounts of plastic deformation, they do not necessarily exhibit high UTS; some highly ductile metals, like gold, are relatively soft.
In real-world applications, the functional strength of a ductile material is often assessed at stress levels below fracture, particularly where permanent but acceptable deformation is allowed. The term proof strength is commonly used to describe the stress at which a small, defined permanent deformation occurs, typically 0.1% or 0.2%. This represents a practical threshold for non-destructive, reversible use in engineering applications.
How Is the Metal Ductility Being Measured?
Ductility is typically measured using two methods, both of which rely on data obtained from tensile testing. While each approach highlights different aspects of deformation, they are not directly interchangeable.
Reduction in Area (RA): This method defines ductility as the percentage decrease in cross-sectional area after fracture compared to the original area. It is calculated using:
Ductility (RA) = [(A₀ − Af) / A₀] × 100%
Where A₀ is the original cross-sectional area, and Af is the area at the point of fracture.
Elongation (Percentage Elongation): This method defines ductility as the percentage increase in gauge length after fracture relative to the original length:
Ductility (Elongation) = [(Lf − L₀) / L₀] × 100%
Where L₀ is the original gauge length, and Lf is the length after fracture.
In both cases, a standardized test specimen is loaded in a tensile testing machine until failure. The machine records force and elongation, allowing a stress-strain curve to be generated. Ductility is observed as the amount of plastic deformation before fracture, typically after the yield point is surpassed. While both measures describe deformation capacity, elongation emphasizes lengthwise deformation, while reduction in area focuses on necking and localized strain.
How Can Ductility Be Improved in Metals?
Ductility in most metals can be improved by annealing. This process requires the metal to be uniformly heated to a temperature that is higher than the glass transition temperature but significantly lower than the softening temperature. This heating causes the crystal boundaries to dissolve, leaving the structure amorphous (when hot). As the metal cools, crystal growth restarts and the structure cools to a maximum general crystal size if the cooling is slow. Ductile behavior will begin to fracture these large crystals into smaller pieces, generally hardening the material. This is commonly called work-hardening, and it leads to brittle behavior and the end of ductility.
What Factors Affect the Ductility of Metals?
Metal ductility is primarily affected by two characteristics:
- A coarse crystal structure promotes ductility.
- A face-centered cubic crystal growth pattern is the most common in ductile metals.
Heat treatment can increase/restore ductility by annealing and remove/reduce it by hot quenching, which freezes a fine crystal structure into the material in some cases.
How Does Temperature Affect the Ductility of Metals?
For most metals, moderate temperature changes have a negligible influence on ductility. It is only as the glass transition temperature for the metal is reached that ductility will begin to increase, as crystal boundaries dissolve and the slip planes are able to move more freely. Some metals, such as lead and antimony, have glass transition temperatures at or below room temperature. They only develop a fixed crystalline structure when chilled. This renders them more ductile.
How are Ductile Metals Used in Engineering?
Ductility is a property that is widely exploited in engineering materials, as an aid to easier processing of complex shapes. All drawn metal wires are the result of the exploitation of ductility, as the metal is pulled to reduce cross-section and increase length. Cold drawing, particularly of seamless stainless steel tubes, is a standard method to extend length in precise ways and induce work hardening. This is the exploitation of ductile behavior to the work-hardening limit. In a less clear-cut way, the ability of metals to bend and flow into shapes, such as in forging or sheet metal pressing, is the exploitation of ductility.
Frequently Asked Questions About Ductile Metals
Which Metal Has the Highest Ductility?
Gold has long been regarded as the most ductile material, with a length of around 3,000 m per gram commonly drawn. However, platinum drawn down using the Wollaston process (encased in silver as a stress shield/distributor) has been drawn to many times this length per gram.
Is Aluminum Ductile Metal?
Yes, aluminum in its pure form is a highly ductile metal, and it can be drawn into very fine wires. As alloying agents increase, the hardness tends to rise and the ductility diminishes, but most aluminum alloys retain fairly high ductility.
Is Zinc Ductile Metal?
Yes, zinc is a highly ductile material when heated to a temperature between 110-150 °C and can be drawn into fine wires. Below this range, it is crystalline and much less ductile; above this range, it begins to weaken and fractures more easily. For more information, see our guide on Brittle Failure.
Is Copper Highly Ductile Metal?
Yes, copper is very ductile and appears on any list of the most ductile metals.
What Is the Difference Between Ductility vs. Malleability
Ductility is the ability of a material to extend under tensile load without fracture. Malleability, on the other hand, is a material’s ability to be formed and distorted under compressive (pressure or impact) loading without displaying internal failure or fracture.
Summary
This article presented ductile metals, explained what they are, and discussed the characteristics of each one To learn more about ductile metals, contact a Xometry representative.
Xometry provides a wide range of manufacturing capabilities and other value-added services for all of your prototyping and production needs. Visit our website to learn more or to request a free, no-obligation quote.
Disclaimer
The content appearing on this webpage is for informational purposes only. Xometry makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through Xometry’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please refer to our terms and conditions for more information.


