Engineers need to know how to calculate thermal conductivity because it can give them valuable insights into materials to help them make the right selection. Mainly, it lets them know if a material is insulating or conductive. In this article, we’ll go over thermal conductivity in more detail, factors that can influence it, and the exact formula for you to calculate it.
What is Thermal Conductivity?
Thermal conductivity refers to how easily heat can move through a material, down a gradient from high temperature to low temperature. If a material is an insulator, you’ll usually find the term “thermal resistivity” used instead. This thermal conductivity/resistivity is often found experimentally. The calculation is a handy way of determining whether a material is an insulator or a conductor. Copper is a highly heat-conductive material, which makes it ideal for something like a heat sink. Ceramic, though, has low thermal conductivity, so it’s perfect for thermal insulation. For heat exchangers, a good thermal conductor will be needed, but something like a furnace lining calls for an insulator.
Thermal conductivity is especially important when it comes to 3D printing. For starters, the bed of the printer needs to be kept hot in order for the first layer to stick to it. 3D printer bed plates are usually made of aluminum plate with a heating element attached to the underside. Aluminum is an excellent heat conductor, so it can evenly distribute the heat wherever it’s needed. Good thermal conductivity is also needed to transfer heat from the thermistor to the plastic in order to melt it. But, on the other hand, if there’s any thermal conductivity inside the extruder assembly, this can cause heat creep, and ultimately, the print can fail.
For heat to move from the hot area to the cold area of a material, a few things happen. First of all, what we perceive as heat when we touch something hot is actually atomic-scale vibrations that happen within the material. When a material absorbs heat energy, that energy is transformed into kinetic energy, causing the atoms to move. But, as atoms in solids don’t have much room to move, they start vibrating, and the ones directly exposed to the heat start crashing into their neighbors. This collision excites the neighbors, and they also begin to vibrate. As this happens and continues to move along from the hot to the cold part of a material, the heat begins to move further down too. It’s kind of like a ripple that spreads from a pebble hitting the surface of a pond.
As conductors, materials with high thermal conductivity can transfer heat from a heat source to a heat sink, keeping equipment cool. They can also move heat from a heat source to cooler fluid in order to heat it up and distribute the heat evenly, which can prevent warping. Materials with low thermal conductivity are good insulators, preventing heat from escaping the source or entering a temperature-sensitive area like inside a spacecraft during atmospheric reentry. However, the calculation is not entirely precise. A material’s thermal conductivity can change with temperature. That’s why some calculations may not be right for you if they were done under different conditions. In addition, thermal conductivity usually only covers heat transfer via conduction and doesn’t really address convection or radiative heat transfer.
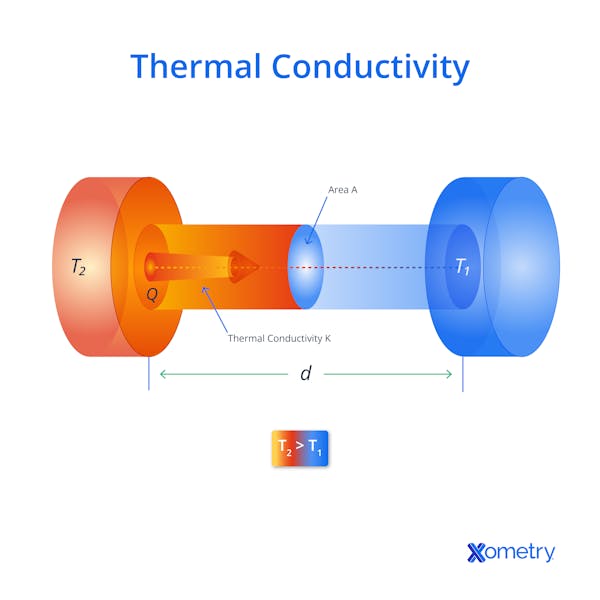
How Does Thermal Conductivity Work?
Thermal conductivity relates to the ability of a material to transfer heat down a gradient from high temperature to low temperature. What we perceive as "heat" when we touch an object is the macroscopic effect of atomic-scale vibrations within the material. When heat energy is absorbed by a material, that energy is transformed into kinetic energy of the atoms. Atoms in solids cannot move around much, so they vibrate. The vibrating atoms directly exposed to the heat energy collide with their neighbors. This transfers the kinetic energy to the neighbors, which then excites atoms even further downstream from the heat source. The vibrations induced by the heat energy move through the material to colder areas, somewhat like a ripple spreading from a pebble hitting the surface of a pond.
What Is the Importance of Thermal Conductivity?
Thermal conductivity is important because it is a measure of how well a material aids (conducts) or resists (insulates against) the flow of heat. In practice, this allows engineers to select the appropriate material for an application. For example, in a heat exchanger, a good thermal conductor is ideal. For a furnace lining, a good insulator is ideal.
What Is the Importance of Thermal Conductivity in 3D Printing?
Thermal conductivity is important for a number of reasons. Firstly, the bed of the 3D printer must be kept hot to ensure that the first layer sticks to it. Bed plates for 3D printing are usually made of aluminum plate with a heating element attached to the underside. Aluminum is a good heat conductor, so a heated aluminum printer bed will transfer heat evenly throughout the print deposition target area.Secondly, thermal conductivity inside the extruder assembly can cause print failures due to heat creep. Finally, thermal conductivity is important as the heat in the hot end must be transferred from the thermistor to the plastic in order to melt it effectively.
How Important Is Thermal Conductivity in Laser Cutting?
Laser cutting and plastic injection molding—both services we offer here at Xometry—are processes in which thermal conductivity plays an important role. For laser cutting, highly thermally conductive materials require more energy in order to cut through them. On the contrary, though, if materials are on the less conductive side, they’ll localize the heat near the cut edge and cause an uneven distribution of heat, which can eventually cause warping or cracking.
How Important Is Thermal Conductivity in Plastic Injection Molding?
Similar things can happen during plastic injection molding. During this process, if the mold is not kept at the best temperature and then cooled down quickly, you can have huge cycle times and run the risk of making a bad-quality part. That’s why molds with high thermal conductivity are great: they allow for quick heating and cooling.
What Is the Formula for Thermal Conductivity?
Without further ado, let’s get straight to the formula for calculating thermal conductivity. First, you’ll need to know what all the symbols mean:
- k = Thermal conductivity
- Q = Heat flux
- A = Cross-sectional area
- ΔT = The difference in temperature of the material’s two sides
- T1 = The material’s hot side
- T2 = The material’s cold side
- d = The length of the material
We’ll use a simplified form of Fourier’s law for heat transfer, but there are a few things to keep in mind with this law. If the temperature on the hot side doesn’t change, heat transfer can be classified as a “steady state,” and heat is only transferred in one direction. Also, the thermal conductivity value of a material will change depending on the temperature. As a general rule, the higher the temperature, the higher the thermal conductivity. So here it is:
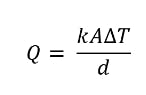
Heat transfer equation.
In this instance, the heat transfer is one-dimensional and in a steady state. We can also rearrange the equation, switching the value for thermal conductivity on the left-hand side. Here’s what the thermal conductivity formula would look like:
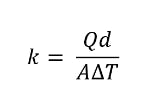
Thermal conductivity equation.
We wouldn’t recommend solely relying on this formula to determine a material’s thermal conductivity, though. The best way to do so is experimentally under controlled conditions, according to strict international standards. Most material data sheets will show thermal conductivity at specific temperatures, or even in a range of temperatures.
To illustrate the effect of a material’s thermal conductivity on the heat flux magnitude, below are three examples of thermal conductivities of common materials that have been experimentally determined. For these examples, the plate is assumed to be 1m thick with a length and breadth of 1m, T1 is equal to 250°C, and T2 is equal to 25°C.
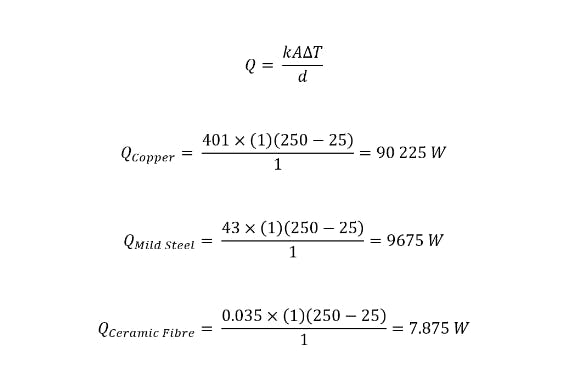
Examples of thermal conductivity calculations for common materials.
What Is the Symbol for Thermal Conductivity?
Thermal conductivity is most commonly represented by the letter k. However, it can also be represented by the Greek letters kappa (κ) and lambda (λ).
What Is the Unit for Thermal Conductivity?
The SI (International System of Units) unit for thermal conductivity is W/m·K, where:
W: Watts
m: Meters
K: Kelvins
In imperial units, thermal conductivity is represented by BTU / (hr·ft·°F), where:
BTU: British Thermal Units
hr: Hours
ft: Feet
°F: Degrees Fahrenheit
How to Calculate the Thermal Conductivity of a Material?
It is not common practice to calculate the thermal conductivity of a material. Instead, thermal conductivity is primarily found through an experimental process that determines the value at controlled conditions at a range of different temperatures. Once the thermal conductivity is known, it can be used to calculate the heat flux, as shown by the formula in Equation 1.
What Are Examples of Calculating Thermal Conductivity?
Thermal conductivity is not calculated but determined via experimental means. However, to illustrate the effect of a material’s thermal conductivity on the heat flux magnitude, three examples are presented below using experimentally determined thermal conductivities of common materials. The plate is assumed to be 1 m thick with a length and breadth of 1 m, and T1 equal to 250 °C and T2 equal to 25 °C.
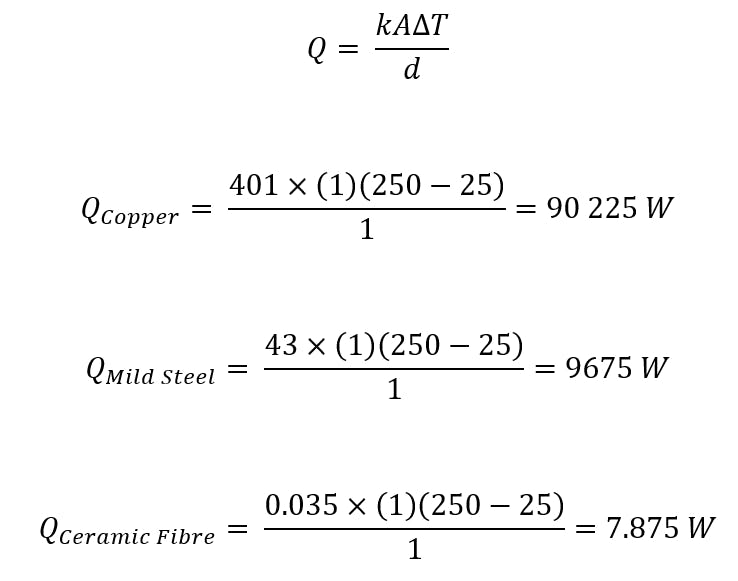
What Are the Factors That Affect the Thermal Conductivity of Materials?
Your calculation with this equation won’t be the same every time, even if you have the same piece of material, and there are several reasons for this:
1. Temperature
Many conductive materials, like metal, tend to decrease in thermal conductivity when they get hot. As they heat up, the atoms and phonons begin to vibrate more intensely, and this reduces the mean free path for free electrons (known as electron phonon-scattering). For nonmetals, the relationship between thermal conductivity and temperature can be more complicated, and the material’s conductivity can either increase or decrease.
2. Density
Materials that are more dense tend to be more thermally conductive. That’s generally because they have a higher packing density of atoms, which helps heat to be transferred via phonons or free electrons.
3. Pressure
It’s possible to increase a material’s density when it’s exposed to high pressure, and this, in turn, can make the material more thermally conductive. Another possibility, however, is that pressure could change the phase of a material—i.e. from a solid to a liquid.
4. Composition
The types of atoms, molecules, or ions in a material can also affect its heat conductivity. Nonmetallic materials, i.e., polymers or ceramics, are less thermally conductive because their molecular structures are more rigid.
5. Structure
The lattice structure of a material could affect the result as some are better at transferring heat than others. Materials with larger crystals are usually better at transferring heat because they have fewer grain boundaries (basically obstacles). The form of the crystal structure can also have an effect. FCC (face-centered cubic) structures like those in copper are more thermally conductive than BCC (body-centered cubic) structures like those found in iron.
6. Porosity
Referring to voids or gas pockets within a material’s structure, porosity can be naturally occurring, added on purpose, or present because of bad processing. The thermal conductivity through these little pockets is much less than the base material. But, ultimately, this will decrease the overall thermal conductivity of that material.
7. Impurities
Any impurities in a material can affect its thermal conductivity, and that’s due to something called electric impurity scattering. These impurities can create local anomalies in the electric potential within the crystal lattice. This can cause the free electronics to move less and ultimately reduce the material’s thermal conductivity.
What Are the Benefits of Thermal Conductivity?
Both materials with very high thermal conductivities and those with very low conductivities can provide benefits to an application, depending on whether heat transfer or heat retention is the more important characteristic. The benefits of using excellent conductors and insulators are listed below:
- Conductors: Materials with high thermal conductivity can efficiently transfer heat from a heat source to a heat sink, keeping equipment cool. Alternatively, conductors can transfer heat from a heat source to cooler fluid in order to heat it up as well as allow for even heat transfer to prevent warping.
- Insulators: Materials with low thermal conductivity can prevent the transfer of heat away from a heat source. This can improve the efficiency of an oven, for example, as it keeps the heat inside where it is needed. Another example would be to keep heat from entering a temperature-sensitive environment like the inside of a spacecraft during atmospheric reentry.
What Are the Limitations of Thermal Conductivity?
Listed below are some limitations of measures of thermal conductivity:
- Not Precise: The thermal conductivity of materials changes with temperature. For that reason, calculations based on thermal conductivity measured under a particular set of conditions may not be accurate when used to estimate heat transfer at other conditions.
- Primarily Conduction Based: Thermal conductivity generally only covers heat transfer via conduction and does not address convection or radiative heat transfer.
What Are Examples of Thermal Conductivity of Different Materials?
In the following table, we list the thermal conductivities of a range of common materials:
| Material | Thermal Conductivity (Cal/cm·s·oC) | Thermal Conductivity (W/m·K) |
|---|---|---|
Material Mild Steel | Thermal Conductivity (Cal/cm·s·oC) 0.102 | Thermal Conductivity (W/m·K) 43 |
Material Type 316 Stainless Steel | Thermal Conductivity (Cal/cm·s·oC) 0.039 | Thermal Conductivity (W/m·K) 16.3 |
Material Copper | Thermal Conductivity (Cal/cm·s·oC) 0.958 | Thermal Conductivity (W/m·K) 401 |
Material Silver | Thermal Conductivity (Cal/cm·s·oC) 1.025 | Thermal Conductivity (W/m·K) 429 |
Material Ceramic Fiber | Thermal Conductivity (Cal/cm·s·oC) 0.00008 | Thermal Conductivity (W/m·K) 0.035 |
Material PLA (FDM 3D Printing Material) | Thermal Conductivity (Cal/cm·s·oC) 0 | Thermal Conductivity (W/m·K) 0.13 |
Material ABS (FDM 3D Printing Material) | Thermal Conductivity (Cal/cm·s·oC) 0.00059 | Thermal Conductivity (W/m·K) 0.25 |
Material SS 316 (SLM 3D Printing Material) | Thermal Conductivity (Cal/cm·s·oC) 0.0389 | Thermal Conductivity (W/m·K) 16.3 |
Material Nylon PA12 (SLS 3D Printing Material) | Thermal Conductivity (Cal/cm·s·oC) 0.00072 | Thermal Conductivity (W/m·K) 0.3 |
Thermal Conductivity Examples in Different Materials
What Does High Thermal Conductivity Mean?
High thermal conductivity refers to the ability of a material to move heat quickly and efficiently. Materials with high thermal conductivity can transfer heat rapidly from one location to another. They are used in applications where fast heat transfer is important, such as in heat exchangers.
Frequently Asked Questions About Thermal Conductivity
What Material Has the Highest Thermal Conductivity?
Diamond has the highest thermal conductivity of any naturally occurring material. This is due to its highly ordered crystal structure as well as the strong covalent bonds between the crystal lattice structure. Diamond has a thermal conductivity of between 2000 to 2500 W/m·K. However, graphene, which is a man-made structure consisting of a planar arrangement of carbon atoms, can have an in-plane heat transfer rate of between 3000 and 5000 W/m·K.
What Does Low Thermal Conductivity Mean?
Low thermal conductivity refers to a material’s inability to conduct heat efficiently. Materials with low thermal conductivity are good insulators. They tend to prevent heat transfer and have fewer free electrons. For that reason, the primary heat transfer mechanism is via lattice or molecular vibration, which is generally less efficient than free electron heat transfer.
What Material Has the Lowest Thermal Conductivity?
Aerogel has the lowest recorded thermal conductivity of any physical material. Aerogel is essentially a gel with micropores filled with gas, typically air. These microvoids create artificial porosity that hinders heat transfer. N-doped graphene-based aerogel can have a thermal conductivity of as low as 0.023 W/m·K. This is about the same heat conductivity as air alone (0.025 W/m-K), and the air is considered a very poor conductor of heat at ambient conditions.
Which Is Better, High or Low Thermal Conductivity?
Whether high or low thermal conductivity is better depends entirely on the application. In heat transfer applications like heat exchangers, high thermal conductivity is ideal, since it improves the rate of heat transfer to the heat transfer fluid. In cases where heat must be prevented from moving to surrounding components like in a furnace, for example, lower thermal conductivity is preferred.
How Xometry Can Help
Xometry provides many manufacturers with services related to metal and thermal conductivity. We offer a wide range of manufacturing capabilities, including 3D printing, laser cutting, CNC machining, and more. If you’d like to learn more about thermal conductivity or request a free, no-obligation quote, reach out to a Xometry representative today.
Check out our Technical Datasheet Glossary to learn more about different material properties.
Disclaimer
The content appearing on this webpage is for informational purposes only. Xometry makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through Xometry’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please refer to our terms and conditions for more information.